Abstract
Multidrug-resistant tuberculosis is an increasing public health concern in many parts of the world, especially in low-income countries, where most cases occur. Traditional drug susceptibility testing is either time-consuming, such as the proportion method on solid media, or expensive, such as the BACTEC 460 system. We have evaluated a new nitrate reductase assay (NRA) that depends on the ability of Mycobacterium tuberculosis to reduce nitrate to nitrite. The reduction can be detected using specific reagents, which produce a color change. We tested a panel of 57 M. tuberculosis strains with various resistance patterns. The bacteria were inoculated on Löwenstein-Jensen medium, either without drugs or with rifampin, isoniazid, streptomycin, or ethambutol and with potassium nitrate (KNO3) incorporated. After incubation for 7, 10, or 14 days, the reagents were added and nitrate reduction, indicating growth, could be detected by a color change. Sensitivities to and specificities for drugs as determined by the NRA method compared to those determined by the BACTEC 460 method were 100 and 100% for rifampin, 97 and 96% for isoniazid, 95 and 83% for streptomycin, and 75 and 98% for ethambutol, respectively. The results were in the majority of the cases available in 7 days. The evaluated method is rapid and inexpensive and could correctly identify most resistant and sensitive M. tuberculosis strains. It has the potential to become an interesting alternative to existing methods, such as the proportion and BACTEC methods, particularly in resource-poor settings.
The spread of multidrug-resistant (MDR) strains of Mycobacterium tuberculosis has become a major public health concern since these bacteria often cause incurable disease, even when expensive second- and third-line drugs are available. Data from 52 countries in the World Health Organization's global project on tuberculosis drug resistance surveillance reported a median prevalence of 1.8% (range, 0 to 18.1%) for MDR strains and 11.1% (range, 2.9 to 40.8%) for strains with any drug resistance (13). Standard methods for drug susceptibility testing (DST) of M. tuberculosis, such as the proportion method, the absolute-concentration method, and the resistance ratio method, are used globally but depend on culture on solid media and are therefore time-consuming (1). The time lag is a significant threat to the patient, the community, and health care workers. The BACTEC 460 method is a radiometric variant of the proportion method (10). It is faster but demands costly equipment and substrate, produces radioactive waste, and is consequently not feasible in most resource-poor settings. Various other techniques, genetic as well as phenotypic, have also been elaborated (2, 6, 7, 8, 9).
There is obviously a great need for fast, reliable, and inexpensive methods for antimicrobial susceptibility testing of M. tuberculosis. The purpose of our study was to evaluate a nitrate reductase assay (NRA) initially developed at the Central Tuberculosis Research Institute in Moscow, Russia (3), where it was called the Griess method, after J.P. Griess, who discovered the chemistry of the detection method used (4). It is based on the ability of M. tuberculosis to reduce nitrate to nitrite, which is routinely used for biochemical identification of mycobacterial species. The presence of nitrite can easily be detected with specific reagents, which produce a color change (5). We have compared the performance of this technique to that of the radiometric BACTEC 460 system in determining the susceptibilities to isoniazid (INH), rifampin (RIF), streptomycin (STR), and ethambutol (EMB) of 57 M. tuberculosis strains. We found a 100% agreement among results for RIF and an overall agreement of 94% by the NRA. The results were in the majority of cases available in 7 days. This technique might become an inexpensive alternative for the rapid and accurate detection of drug-resistant M. tuberculosis strains.
(Preliminary results were presented at the 22nd Annual Congress of the European Society of Mycobacteriology, Berlin, Germany, 1 to 4 July 2001.)
MATERIALS AND METHODS
Strains.
A panel of 56 clinical isolates of M. tuberculosis, 22 of which were fully susceptible and 34 of which had different resistance patterns, from the strain collection at the Swedish Institute for Infectious Disease Control and the susceptible reference strain M. tuberculosis H37Rv (ATCC 25618) were used. Drug-resistant clinical isolates originated from different parts of the world (Sweden, Estonia, Russia, China, Nepal, Honduras, Ethiopia, and Somalia), whereas most fully susceptible strains were clinical isolates from Sweden. All strains were stored at −70°C and cultured on standard Löwenstein-Jensen medium before use.
Radiometric DST.
Antibiotic stock solutions for INH, RIF, STR, and EMB were prepared in advance and kept at−70°C until use. Drug susceptibility was determined by standard procedures in a BACTEC 460 instrument (Becton Dickinson, Sparks, Md.) (10) with the following critical concentrations: 0.2 μg/ml for INH, 2.0 μg/ml for RIF, 4.0 μg/ml for STR, and 5.0 μg/ml for EMB.
NRA DST.
The NRA was performed as described by Golyshevskaia et al. (3) but with modifications regarding critical antibiotic concentrations, size of the inoculum, and the number of growth controls. In short, we used standard Löwenstein-Jensen medium, with 1,000 μg of potassium nitrate (KNO3)/ml and with or without antimicrobials incorporated. The following critical concentrations were used: 0.2 μg/ml for INH, 40 μg/ml for RIF, 4.0 μg/ml for STR, and 2.0 μg/ml for EMB. These are the antibiotic concentrations recommended for DST by the proportion method on Löwenstein-Jensen medium (1). Golyshevskaia et al. (3) used other critical concentrations for INH (1.0 μg/ml) and STR (10.0 μg/ml), but in our pilot study (data not shown), these concentrations appeared to be too high. The medium was prepared in 10-ml portions in 75- by 25-mm screw-cap glass tubes. For NRA testing, two 1-μl loops of bacteria, from fresh cultures on Löwenstein-Jensen medium, were dispensed and vortexed in 3 ml of phosphate-buffered saline (pH 7.4) in 7.5-ml screw-cap bottles containing a few 3-mm-diameter glass beads, in order to obtain approximately the turbidity of McFarland standard no. 1. Part of the suspension was diluted 1:10 in phosphate-buffered saline. For each strain, 0.2 ml of the undiluted suspension was inoculated into the tubes containing Löwenstein-Jensen medium with KNO3 and the antibiotics and 0.2 ml of the 1:10 dilution was inoculated into three drug-free tubes containing Löwenstein-Jensen medium with KNO3 incorporated. The latter tubes served as growth controls. The tubes were incubated at 37°C, and 0.5 ml of a mixture of three reagents was added to one drug-free control tube after 7 days. If any color change (strong or weak) could be seen, the corresponding antibiotic-containing tubes were also tested and susceptibility results were read. If no color change was seen in the growth control tube, this tube was discarded and the other two control tubes and the antibiotic tubes were reincubated. The procedure was then repeated at day 10, using the second growth control, and if needed, also at day 14, using the last growth control tube. The reagents consisted of 1 part 50% (vol/vol) concentrated hydrochloric acid (HCl), 2 parts 0.2% (wt/vol) sulfanilamide, and 2 parts 0.1% (wt/vol) n-1-naphthylethylenediamine dihydrochloride (5). They were mixed shortly before use. The results were classified as negative (no color change) or ± (pale pink) to 5+ (deep red to violet). An isolate was considered resistant to a certain drug if there was a color change in the antibiotic tube in question greater than that in the 1:10-diluted growth control on the same day. All strains were coded and tested blindly. Comparison of NRA and BACTEC 460 results was done after decoding.
RESULTS
Testing of 57 M. tuberculosis strains for their susceptibilities to INH, RIF, STR, and EMB gave an overall agreement of 94% between the NRA and BACTEC 460 techniques (215 of 228 susceptibility tests) (Table 1). The sensitivity of the NRA compared to that of the BACTEC 460 method was found to be 95% (93 of 98 tests), and the specificity was found to be 94% (122 of 130 tests). Complete agreement was found in results for RIF. A good correlation was also found for INH, while some problems were encountered when we tested for specificity for STR resistance and sensitivity in detecting EMB resistance (Table 1). Results were completely concordant for 45 of 57 strains, whereas results were discordant for 12 strains in 13 tests. The NRA was easy to read; an example of results for susceptible and resistant strains can be seen in Fig. 1. Results were available in 7 days for 61% of the strains, in 10 days for 87% of the strains, and in 14 days for 100% of the strains. The fully susceptible strains were significantly more likely to be ready in 7 days than strains with any resistance (P = 0.023) (Yates' corrected chi-square test).
TABLE 1.
Susceptibility results, sensitivity, and specificity of the NRA method compared to those of the BACTEC 460 method for M. tuberculosis
| Drug | BACTEC 460 determination | NRA result
|
|||
|---|---|---|---|---|---|
| No. of resistant strains | No. of susceptible strains | % Sensitivity | % Specificity | ||
| RIF | Resistant | 31 | 0 | 100 | 100 |
| Susceptible | 0 | 26 | |||
| INH | Resistant | 32 | 1 | 97 | 96 |
| Susceptible | 1 | 23 | |||
| STR | Resistant | 21 | 1 | 95 | 83 |
| Susceptible | 6 | 29 | |||
| EMB | Resistant | 9 | 3 | 75 | 98 |
| Susceptible | 1 | 44 | |||
| Total | Resistant | 93 | 5 | 95 | 94 |
| Susceptible | 8 | 122 | |||
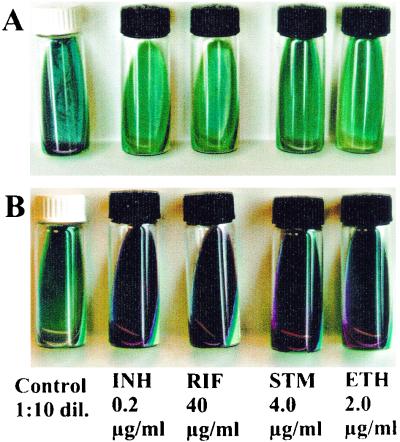
FIG. 1.
Examples of NRA results. A strain is considered resistant to a certain drug if there is a color change in the drug-containing tube greater than that in the 1:10-diluted (1:10 dil.) control tube. (A) Fully susceptible strain; (B) strain resistant to all four tested drugs.
DISCUSSION
Complete agreement between the results of the NRA and BACTEC 460 methods was found for RIF, which is important, since RIF, together with INH, is the most important antituberculosis drug. Resistance to RIF is also almost always associated with multidrug resistance (11) and can thus serve as a marker of MDR M. tuberculosis strains if resources are limited. The NRA seems to be comparable to the BACTEC 460 system in detecting INH resistance (sensitivity, 97%; specificity, 96%), while the corresponding figures for STR (95 and 83%, respectively) and EMB (75 and 98%, respectively) were rather less impressive. However, the susceptibility of M. tuberculosis to STR and EMB is somewhat more difficult to determine accurately, even by conventional standard methods (13).
The NRA method utilizes the detection of nitrate reduction as an indication of growth, and therefore, results can be obtained faster than by visual detection of colonies. The tests were ready in 7 days for a majority of the isolates, which is comparable with the time required by the BACTEC 460 method and clearly faster than the proportion method on solid media, which takes 3 to 4 weeks.
The ability to reduce nitrate is typical for M. tuberculosis, although some other mycobacterial species, like Mycobacterium kansasii, and most rapid growers share this characteristic (5). Nitrate reductase-negative strains of M. tuberculosis are rare (<1%) (5) and would create no false results since the control would be negative and the test would therefore be invalid. No such strains were encountered in our study. Strains of Mycobacterium bovis do not reduce nitrate (5), for which reason the NRA technique is not applicable to DST of them.
Another possible limitation is that nitrite might be further reduced to nitric oxide, which cannot be detected by the reagents used. When the nitrate reduction test is performed for the purpose of species identification, zinc powder is added to all negative tubes (5). Zinc reduces nitrate rapidly, and a true negative test will directly turn red, while there will be no color change in a tube where reduction has passed beyond nitrite. In our pilot experiments, we added zinc to all negative tubes, but since the result was always confirmed and since the peak in nitrate reductase activity of M. tuberculosis takes place after 3 to 4 weeks of incubation (12), this step was omitted. Further studies will clarify the role of zinc powder in the NRA.
Existing methods for DST are either time-consuming, as is the proportion method on solid media, or expensive, as is the BACTEC 460 method. Several alternative methods have been proposed. Molecular genetic methods such as the line probe assay (Innogenetics, Ghent, Belgium) (9) are fast but far too expensive to be used in most resource-poor settings and have been developed mainly for RIF susceptibility testing. Automated test systems, for example, MB/BacT (Organon Teknika, Boxtel, The Netherlands) (2) and the Mycobacteria Growth Indicator Tube (Becton Dickinson) (8), are rapid and easy to use but require heavy investments in equipment and running costs. Colorimetric assays based on the reduction of dyes, i.e., Alamar Blue (7) or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (6), have been tested with some success. However, the application of liquid media in a microplate format is complicated and might be a biohazard and the risk of cross-contamination is not negligible.
The NRA is rapid, inexpensive, and easy to perform. It requires neither fancy equipment nor expensive substrates or reagents. Nevertheless, more studies are needed to further assess accuracy and reproducibility before this method can be considered for clinical use. We have initiated a field study in Honduras in order to analyze its applicability in a resource-poor setting. It might also be possible to develop a direct test which, applied directly to microscopy-positive sputa, would drastically reduce the time needed for detection of drug-resistant M. tuberculosis. We believe that the NRA can become an important tool for rapid detection of MDR M. tuberculosis strains worldwide.
Acknowledgments
We thank Valyentina Golyshevskaia and her colleagues at the Central Tuberculosis Research Institute in Moscow, Russia, for introducing us to this technique and Björn Petrini at the Karolinska Hospital tuberculosis laboratory for valuable comments on the manuscript.
Financial support from the Swedish International Development Cooperation Agency/Department for Research Cooperation (Sida/SAREC) is gratefully acknowledged.
REFERENCES
- 1.Canetti, G., S. Froman, J. Grosset, P. Hauduroy, M. Langerová, H. T. Mahler, G. Meissner, D. A. Mitchison, and L. Sula. 1963. Mycobacteria. Laboratory methods for testing drug sensitivity and resistance. Bull. W. H. O. 29:565-578. [PMC free article] [PubMed] [Google Scholar]
- 2.Díaz-Infantes, M. S., M. J. Ruiz-Serrano, L. Martínez-Sanchez, A. Ortega, and E. Bouza. 2000. Evaluation of the MB/BacT mycobacterium detection system for susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 38:1988-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golyshevskaia, V. I., A. A. Korneev, L. N. Chernousova, L. G. Selina, T. A. Kazarova, T. D. Grishina, S. G. Safonova, V. A. Puzanov, G. M. Nikolaeva, and N. I. Fadeeva. 1996. New microbiological techniques in diagnosis of tuberculosis. Probl. Tuberk. 6:22-25. [PubMed] [Google Scholar]
- 4.Griess, J. P., and Z. A. H. H. Bemerkungen. 1879. Über einige Azoverbindungen. Ber. Deutch Chem. Ges. 12:426-428.
- 5.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory, p. 96-103. U.S. Department of Health and Human Services, Atlanta, Ga.
- 6.Mshana, R. N., G. Tadesse, G. Abate, and H. Miörner. 1998. Use of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1214-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palomino, J. C., and F. Portaels. 1999. Simple procedure for drug susceptibility testing of Mycobacterium tuberculosis using a commercial colorimetic assay. Eur. J. Clin. Microbiol. Infect. Dis. 18:380-383. [DOI] [PubMed] [Google Scholar]
- 8.Palomino, J. C., H. Traore, K. Fissette, and F. Portaels. 1999. Evaluation of Mycobacteria Growth Indicator Tube (MGIT) for drug susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 3:344-348. [PubMed] [Google Scholar]
- 9.Rossau, R., H. Traore, H. De Beenhouwer, W. Mijs, G. Jannes, P. De Rijk, and F. Portaels. 1997. Evaluation of the INNO-LiPA Rif. TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob. Agents Chemother. 41:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vareldzis, B. P., J. Grosset, I. De Kantor, J. Crofton, A. Laszlo, M. Felten, M. C. Raviglione, and A. Kochi. 1994. Drug-resistant tuberculosis: laboratory issues. World Health Organization recommendations. Tuber. Lung Dis. 75:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen, S. 1960. A study of nitrate reduction by mycobacteria. Acta Tuberc. Scand. Suppl. 48:78-82. [PubMed] [Google Scholar]
- 13.World Health Organization. 2000. The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. Anti-tuberculosis drug resistance in the world, report no. 2: prevalence and trends. World Health Organization, Geneva, Switzerland.