Abstract
Autoimmune pancreatitis is a chronic inflammatory disorder that is often misdiagnosed as pancreatic cancer. Since autoimmune pancreatitis is benign and responds to steroid management, it is important to diagnose it to avoid unnecessary surgical intervention. We describe a novel case of IgG4-associated autoimmune pancreatitis presenting with tubulointerstitial nephritis as renal lesions mimicking metastatic tumours but with no change in renal function.
A 52-year-old previously healthy man experienced a 30-pound weight loss over 2 months. The patient was newly diagnosed with diabetes mellitus, and an abdominal ultrasound identified a pancreatic mass. Clinical history included crampy abdominal discomfort localized to the right lower quadrant, which had started 2 months earlier. No other symptoms suggestive of biliary obstruction or pancreatic insufficiency were present. Past medical history included asthma but was otherwise unremarkable, with no history of alcohol abuse or drug exposure. Findings on physical examination were normal, as were all results of initial laboratory studies, including lipase and liver enzyme levels, liver function, urinalysis results, creatinine level (66 μmol/L), levels of tumour markers (cancer antigen 19–9, carcinoembryonic antigen) and complement levels.
A CT scan of the abdomen showed a bulky and heterogeneous mass in the pancreatic head, neck and uncinate (Fig. 1A), with encasement of the superior mesenteric vein (Fig. 1B). Although multiple retroperitoneal lymph nodes were identified, none was enlarged enough to fulfill the size criteria for metastasis. Three solid lesions were noted in the left kidney, with the largest measuring 1.7 cm in diameter; 3 lesions were identified in the right kidney, with the largest measuring 1.5 cm. The contrast-enhanced scans demonstrated that the lesions did not represent hyperdense cysts (Fig. 1C). A subsequent MRI confirmed the CT findings.
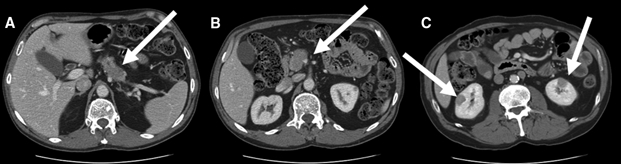
Fig. 1: A: Bulky, heterogenous mass in pancreatic head (arrow). B: Narrowing and pinching of superior mesenteric vein (arrow). C: Bilateral renal lesions (arrows) appearing as well-circumscribed masses mimicking tumours.
An endoscopic ultrasound-guided fine-needle biopsy of the pancreatic mass was performed, and cytology of the aspirate revealed no malignant cells. However, we felt that the diagnosis of pancreatic cancer could not be completely ruled out, so we performed a percutaneous biopsy of the pancreatic lesion. Needle-core biopsies of the pancreas demonstrated morphology suggestive of autoimmune pancreatitis. The pancreatic tissue was almost completely replaced with fibrous tissue and an inflammatory infiltrate composed of lymphocytes and plasma cells, which were positive for IgG4 (Fig. 2A and B). A biopsy of the duodenum revealed duodenitis with loss of mucosal villi and extensive lymphoplasmacytic and eosinophilic infiltration, which stained positive for IgG4.
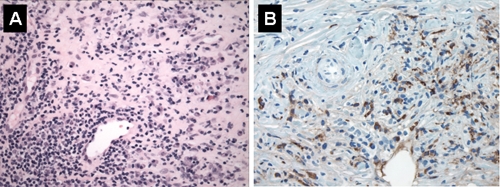
Fig. 2: A: Pancreatic tissue showing extensive lymphoplasmacytic inflammatory infiltrate with areas of fibrosis and sclerosis; no normal pancreatic parenchyma is visible (hematoxylin–eosin stain, original magnification × 400). B: Many inflammatory cells are immunoreactive for IgG4 (immunoperoxidase, original magnification × 400).
Laparoscopic resection was performed of one of the renal lesions, which proved to be non-neoplastic and revealed chronic tubulointerstitial nephritis with extensive interstitial fibrosis. As with the previous biopsies, there was diffuse inflammatory lymphoplasmacytic and eosinophilic infiltrate in the interstitium, which resulted in tubular obliteration (Fig. 3A and B). No microorganisms or viral inclusions were identified. On immunochemistry there was a mixture of T and B lymphocytes; plasma cells marked uniformly for IgG and IgG4 and showed no light-chain restriction. Subsequent laboratory studies revealed elevated serum IgG and IgG4 levels. Serum electrophoresis demonstrated a slightly elevated gamma globulin level, while rheumatoid factor and antinuclear antibody levels were normal. No fresh renal tissue was available for immunofluoresence to determine whether antitubular basement membrane antibodies were present.
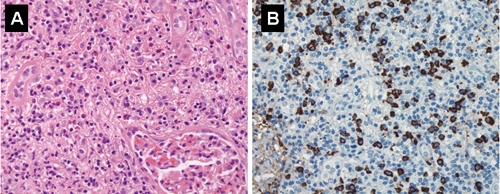
Fig. 3: A: Renal lesion showing extensive lymphoplasmacytic inflammatory infiltrate with scattered eosinophils; note interstitial fibrosis and almost complete loss of tubules (hematoxylin–eosin stain, original magnification × 400). B: Many inflammatory cells stain positive for IgG4 (immunoperoxidase, original magnification × 400).
We initiated a treatment regimen for a presumed diagnosis of autoimmune pancreatitis with prednisone (40 mg/d) for 4 weeks. A follow-up CT scan of the abdomen revealed a normal-sized pancreas with complete resolution of the swelling (Fig. 4A and B). The largest renal lesion decreased in size, and the remaining lesions were not identifiable on the repeat CT scan (Fig. 4C).
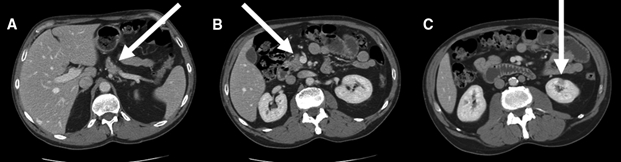
Fig. 4: A: Resolution of swelling of mass in pancreatic head after treatment (arrow). B: Mass is released from the superior mesenteric vein, revealing a visible plane (arrow). C: Complete resolution of lesions in right kidney after treatment (arrow), with significant decrease of lesions in left kidney.
At 13 months' follow-up the patient was asymptomatic after having been off the steroid therapy for 5 months. However, a CT scan demonstrated a recurrence, since the pancreatic body was slightly enlarged and the renal lesions had increased in size. The patient was given azathioprine (75 mg once daily) for 6 months to induce and sustain a response.
Follow-up CT and MRI scans performed at 22 months demonstrated a normal-looking pancreas and stable renal lesions. The patient was free of disease, and results of all laboratory studies, including creatinine level, urinalysis results and levels of IgG and IgG4 and other autoimmune markers, were normal.
Comments
Although tubulointerstitial nephritis has recently been described for the first time in association with autoimmune pancreatitis,1,2 to our knowledge this is the first case of tubulointerstitial nephritis in association with autoimmune pancreatitis mimicking a tumour of the kidney without significantly impairing renal function. This is significant because it may be misdiagnosed as pancreatic cancer metastases. The resolution of the pancreatic and kidney lesions after corticosteroid therapy strongly suggests an underlying systemic autoimmune process.
Autoimmune pancreatitis is a chronic inflammatory disorder hypothesized to be induced from autoantibodies directed toward the pancreatic duct epithelium.3 It was first described in 1961 by Sarles and colleagues as a case of pancreatitis with hypergammaglobulinemia.4 Thirty-five years later, in 1996, Kino-Ohsaki and colleagues shed light onto its pathogenesis, demonstrating that carbonic anhydrase II, an enzyme located in pancreatic duct epithelium, is a target of the acquired autoantibodies.5 Recently, Hamano and colleagues6 reported that elevated concentrations of IgG4 were closely associated with autoimmune pancreatitis. Okazaki and colleagues7 demonstrated that anti-lactoferrin antibody, antinuclear antibody and rheumatoid factor may also be elevated. Kamisawa and colleagues were the first to postulate that autoimmune pancreatitis may be part of an IgG4-positive systemic autoimmune disease characterized by IgG4 plasma cell deposition in various organs, including the pancreas, the portal area of the liver, the lungs, the kidneys, gastric and colonic mucosa and bone marrow.8 Although the exact role of these antibodies has yet to be confirmed, the underlying inflammation produces pancreatic swelling, which is often misdiagnosed as pancreatic cancer. Since the management of autoimmune pancreatitis is medical, in contrast to the surgical treatment of adenocarcinoma, an accurate diagnosis is important to avoid an unnecessary pancreatic resection.
Although the exact incidence of autoimmune pancreatitis is unknown, a recent review reported the overall incidence to be from 1.9% to 6.6%.9 We know of only 1 case in North America that was diagnosed before surgery.10 The majority of the documented cases originate from Japan, Korea and Europe. Most cases in North America have been reported after pancreaticoduodenectomy because of a preoperative diagnosis of pancreatic cancer. Recently, 2 large patient series reported that 2.3% of pancreaticoduodenectomies revealed a misdiagnosed case of autoimmune pancreatitis.11,12
The diagnosis of autoimmune pancreatitis is difficult, since the clinical presentation and findings on abdominal imaging often mimic pancreatic cancer. Both disorders affect similar age groups (60–65 years) and show slight male predilection (64%–68%).11,12 The most common symptoms are also similar and include vague abdominal pain, weight loss, jaundice, night sweats, new-onset diabetes and steatorrhea. On radiographic imaging, a mass in the head of the pancreas is the most common finding in both disorders.
Despite the similar presentation, certain clinical features favour a diagnosis of autoimmune pancreatitis and warrant further investigation, in particular the presence of other autoimmune disorders such as primary sclerosing cholangitis, Sjögren's syndrome, diabetes mellitus and tubulointerstitial nephritis.13 A fluctuating course of jaundice was found in 33% of patients with autoimmune pancreatitis.14 Radiographically, diffuse glandular enlargement with delayed enhancement of the swollen pancreatic parenchyema is often identified, and endoscopic retrograde cholangiopancreatography often demonstrates segmental and diffuse narrowing of the main pancreatic duct.14,15
When autoimmune pancreatitis is suspected, tissue biopsy remains the “gold standard” for diagnosis. Histologically, the condition is characterized by dense periductal lymphoplasmacytic infiltration, periductal and parenchymal fibrosis and obliterative venulitis.16 Measurement of the serum IgG4 level should be considered when differentiating between autoimmune pancreatitis and pancreatic adenocarcinoma, since it has a 97% accuracy, 95% sensitivity and 97% specificity.6
Autoimmune pancreatitis responds well to corticosteroid treatment.13,17,18 Suggested indications for steroid therapy include obstructive jaundice and the presence of associated autoimmune disorders, such as diabetes mellitus.18 The recommended treatment regimen is a starting dose of 30–40 mg/d of prednisone until symptoms improve, followed by dose tapering of 5 mg/w.13,14 A follow-up CT scan can be performed to document pancreatic size reduction and to ensure that pancreatic cancer has not been misdiagnosed as autoimmune pancreatitis. If the symptoms recur, subsequent abdominal imaging and repeat tissue biopsy should clarify the diagnosis.
Footnotes
This article has been peer reviewed.
Contributors: All of the authors contributed to the manuscript through data collection and manuscript development and have approved the final version to be published.
Competing interests: None declared.
Correspondence to: Elijah Dixon, University of Calgary, Tom Baker Cancer Centre, 1331 29th St. NW, Calgary AB T2N 4N2; fax 403 283-1651; elijah.dixon@calgaryhealthregion.ca
REFERENCES
- 1.Takeda S, Haratake J, Kasai T, et al. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant 2004;19:474-6. [DOI] [PubMed]
- 2.Uchiyama-Tanaka Y, Mori Y, Kimura T, et al. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis 2004;43:e18-25. [DOI] [PubMed]
- 3.Cavallini G. Is chronic pancreatitis a primary disease of the pancreatic ducts? A new pathogenetic hypothesis. Ital J Gastroenterol 1993;25:391-6. [PubMed]
- 4.Sarles H, Sarles JC, Muratore R, et al. Chronic inflammatory sclerosis of the pancreas — An autonomous pancreatic disease? Am J Dig Dis 1961;6:688-98. [DOI] [PubMed]
- 5.Kino-Ohsaki J, Nishimori I, Morita M, et al. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjogren's syndrome. Gastroenterology 1996;110:1579-86. [DOI] [PubMed]
- 6.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732-8. [DOI] [PubMed]
- 7.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 2000;118:573-81. [DOI] [PubMed]
- 8.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003;38:982-4. [DOI] [PubMed]
- 9.Deshpande V, Mino-Kenudson M, Brugge W, et al. Autoimmune pancreatitis: more than just a pancreatic disease? A contemporary review of its pathology. Arch Pathol Lab Med 2005;129:1148-54. [DOI] [PubMed]
- 10.Fernandez-del Castillo CF, Sahani DV, Lauwers GY. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 27-2003. A 36-year-old man with recurrent epigastric pain and elevated amylase levels. N Engl J Med 2003;349:893-901. [DOI] [PubMed]
- 11.Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, et al. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis [discussion 858-9]. Ann Surg 2003;237:853-8. [DOI] [PMC free article] [PubMed]
- 12.Weber SM, Cubukcu-Dimopulo O, Palesty JA, et al. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma [discussion 137-9]. J Gastrointest Surg 2003;7:129-37. [DOI] [PubMed]
- 13.Gelrud A, Freedman SD. Autoimmune pancreatitis. J Gastrointest Surg 2005;9:2-5. [DOI] [PubMed]
- 14.Kamisawa T, Egawa N, Nakajima H, et al. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol 2003;98:2694-9. [DOI] [PubMed]
- 15.Horiuchi A, Kawa S, Hamano H, et al. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc 2002;55:494-9. [DOI] [PubMed]
- 16.Klimstra DS, Adsay NV. Lymphoplasmacytic sclerosing (autoimmune) pancreatitis. Semin Diagn Pathol 2004;21:237-46. [DOI] [PubMed]
- 17.Kim KP, Kim MH, Song MH, et al. Autoimmune chronic pancreatitis. Am J Gastroenterol 2004;99:1605-16. [DOI] [PubMed]
- 18.Kamisawa T, Yoshiike M, Egawa N, et al. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study [discussion 238-40]. Pancreatol 2005;5:234-8. [DOI] [PubMed]


