Abstract
Tiamulin is a pleuromutilin derivative used in veterinary practice for the control and specific therapy of infections in swine. This report summarizes studies to establish standardized susceptibility testing methods, interpretive criteria, and reagent details for use in veterinary methods recently developed by the National Committee for Clinical Laboratory Standards (NCCLS) (standards M31-A and M37-A, NCCLS, Wayne, Pa., 1999). A total of 636 fastidious and nonfastidious animal and human pathogens were processed by using media and procedures described by the NCCLS. Tiamulin disk diffusion tests used a 30-μg disk concentration, and the proposed MIC breakpoints corresponding to levels achievable in animal target tissues (lung) were ≤4 μg/ml for susceptibility and ≥32 μg/ml for resistance. Correlate zone diameters for specific nonfastidious species were as follows: for Pasteurella multocida and staphylococci tested on Mueller-Hinton agar, susceptibility at ≥19 mm and resistance at ≤11 mm, and for Actinobacillus suis, Erysipelothrix rhusiopathiae, and Streptococcus suis tested on enriched chocolate Mueller-Hinton agar, susceptibility at ≥16 mm and resistance at ≤8 mm. When Actinobacillus pleuropneumoniae was tested, a susceptibility breakpoint of ≤16 μg/ml (≥9 mm) was suggested for veterinary fastidious medium broth and enriched chocolate Mueller-Hinton agar. Absolute categorical agreement between NCCLS dilution and disk diffusion test results with these criteria ranged from 90.5 to 96.2%. Tiamulin susceptibility testing methods appear to be accurate in their categorical classification for indicated species, and their availability will allow immediate testing of animal isolates to guide therapy via appropriate levels of dosing and to monitor the development of resistance for agents in this unique class.
Tiamulin is a pleuromutilin derivative antimicrobial used in the control and treatment of veterinary gram-positive and gram-negative pathogens, with a particular emphasis on infections in swine (11). It has exceptional activity (MIC, ≤1 μg/ml) against anaerobic bacterial species, intestinal spirochetes, and Mycoplasma spp. (3, 12, 13). The present study was initiated in order to document the sustained antimicrobial activity of tiamulin against indicated bacterial species and rapidly growing and fastidious animal pathogens and to establish choices of diagnostic reagents. MIC and disk diffusion zone diameter correlates were determined in order to ensure the precision and accuracy of the susceptibility testing procedures performed according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines for susceptibility testing of veterinary antimicrobial agents (5, 6).
MATERIALS AND METHODS
Organisms tested.
The organisms tested were isolates from various types of infections and included both human and veterinary pathogens. A total of 636 strains were processed, each identified by routine methods at the institution of initial isolation and confirmed by reference testing at a referral location (CAST Laboratories, Iowa City, Iowa; Iowa State University, Ames). These strains included 392 nonfastidious bacteria (Table 1) : Aerococcus spp. (3 strains), Enterococcus spp. (71 strains), Staphylococcus aureus (150 strains), coagulase-negative staphylococci (CoNS) (99 strains), Streptococcus spp. (4 strains), Enterobacteriaceae (24 strains; 8 species), Acinetobacter baumannii (2 strains), Pseudomonas aeruginosa (6 strains), Stenotrophomonas maltophilia (2 strains), and Pasteurella multocida (31 strains). Also tested were 244 fastidious veterinary pathogens (Table 2) : Actinobacillus pleuropneumoniae (170 strains), Actinobacillus suis (21 strains), Erysipelothrix rhusiopathiae (11 strains), Haemophilus parasuis (18 strains), and Streptococcus suis (24 strains).
TABLE 1.
Tiamulin activity as demonstrated by MIC and zone diameter of inhibition results tested against 392 nonfastidious bacteria
| Organism (no. of strains tested) | MIC (μg/ml)
|
Median zone diameter (mm) | Source | |
|---|---|---|---|---|
| 50% | 90% | |||
| Gram-positive cocci | ||||
| Aerococcus spp. (3) | 32 | 9 | Animal | |
| Enterococcus spp. (71)a | >32 | >32 | 6 | Animal and human |
| S. aureus (150) | 1 | 2 | 29 | Animal and human |
| CoNS (99)b | ≤0.5 | 1 | 34 | Human |
| Streptococcus spp. (4)c | >32 | 6 | Animal | |
| Enterobacteriaceae | ||||
| Citrobacter freundii (2) | >32 | 9 | Animal | |
| Enterobacter spp. (4)d | >32 | 6 | Animal | |
| Escherichia coli (5) | >32 | 7 | Animal | |
| Klebsiella pneumoniae (3) | >32 | 6 | Animal | |
| Salmonella (10)e | >32 | >32 | 6 | Animal and human |
| Nonfermenters | ||||
| A. baumannii (2) | >32 | 12 | Human | |
| P. aeruginosa (6) | >32 | 6 | Human | |
| S. maltophilia (2) | >32 | 6 | Human | |
| P. multocida (31) | 16 | 32 | 14 | Animal |
Includes E. avium (3 strains), E. casseliflavus (3 strains), E. durans (6 strains), E. faecalis (20 strains), E. faecium (25 strains), E. gallinarum (1 strain), E. hirae (9 strains), and Enterococcus species (4 strains).
Includes S. auricularis (6 strains), S. capitus (6 strains), S. epidermidis (50 strains), S. haemolyticus (19 strains), S. hominis (6 strains), S. saprophyticus (6 strains), and S. warneri (6 strains).
Includes S. bovis (1 strain), S. equinus (1 strain), and S. uberis (2 strains).
Includes E. aerogenes (two strains) and E. cloacae (two strains).
Includes Salmonella enterica serovar Choleraesuis (two strains), Salmonella species (five strains), and S. enterica serovar Typhimurium (three strains).
TABLE 2.
Tiamulin activity as demonstrated by MICs and zone diameters of inhibition for 244 fastidious veterinary pathogens with three broth medium formulations
| Organism | Broth medium | No. of strains testeda | MIC (μg/ml)
|
Median zone diameter (mm)b | |
|---|---|---|---|---|---|
| 50% | 90% | ||||
| A. pleuropneumoniae | VFM | 170 | 8 | 16 | 12 |
| HTM | 170 | 8 | 16 | ||
| LHB | NG | ||||
| A. suis | VFM | 21 | 16 | 16 | 14 |
| HTM | 21 | 16 | 16 | ||
| LHB | 21 | 8 | 16 | ||
| E. rhusiopathiae | VFM | 11 | 2 | 4 | 32 |
| HTM | 11 | 1 | 8 | ||
| LHB | 11 | 1 | 2 | ||
| H. parasuis | VFM | NG | |||
| HTM | 18 | 2 | 8 | 20 | |
| LHB | NG | ||||
| S. suis | VFM | 24 | 1 | 16 | 18 |
| HTM | 24 | 2 | 16 | ||
| LHB | 24 | 2 | 16 | ||
NG, no growth.
Disk diffusion testing performed on CMHA with a 30-μg tiamulin disk.
Susceptibility testing.
Tiamulin, as hydrogen fumarate standard powder, was provided by Boehringer Ingelheim Vetmedica, Inc. (St. Joseph, Mo.). Broth microdilution testing of tiamulin was performed with four different media formulations: nonfastidious bacteria were tested in cation-adjusted Mueller-Hinton broth, whereas the fastidious veterinary pathogens were each tested in veterinary fastidious medium (VFM), haemophilus test medium (HTM), and Mueller-Hinton broth supplemented with 5% lysed horse blood (LHB) (5, 7). Tiamulin was dispensed into broth microdilution trays in a serial twofold concentration range of 0.25 to 32 μg/ml. All trays were held at −20°C or below until used. The trays were brought to room temperature before inoculation (5 × 105 CFU/ml), and the technical details of the veterinary NCCLS (5, 7) documents were followed.
The 30-μg tiamulin disks were produced by BD Microbiology Systems (Cockeysville, Md.), and these reagents were used following NCCLS methods M2-A7 (8) and M31-A (5). The nonfastidious isolates were tested on Mueller-Hinton agar (MHA), and the fastidious isolates were tested on chocolate MHA (CMHA). The fastidious organisms were tested using incubation conditions of 35°C in 5 to 7% CO2 for 20 to 24 h and the nonfastidious organisms were tested in ambient air at 35°C for 16 to 18 h. The MIC endpoints and zone diameters of inhibition were read as defined by the NCCLS documents (5, 7, 8). The results were compared by scattergram, regression statistics, and error-rate bounding analysis. Breakpoints for susceptibility were proposed based on available tiamulin pharmacokinetic data (Boehringer Ingelheim Vetmedica, Inc., data on file) and NCCLS (6, 9) guidelines. Quality control was performed with A. pleuropneumoniae ATCC 27090 and S. aureus ATCC 25923 tested against enrofloxacin; all results were within MIC and zone diameter limits established by Marshall et al. (4), NCCLS (5), and the reagent manufacturer.
In addition, preliminary experiments were performed to determine the optimal drug disk content by using investigator-prepared disks containing 5, 15, 30, 60, and 90 μg of tiamulin. Each disk was tested (10 replicates) against the NCCLS quality control strains: Enterococcus faecalis ATCC 29212 and S. aureus ATCC 25923, 29213, and 43300 (methicillin resistant). A 15-μg erythromycin disk was used as a control drug. Furthermore, Neo-Sensitabs (Rosco Diagnostica, Taastrup, Denmark) at 30 μg were tested against control strains with ranges 1 to 2 mm larger than those recorded for the corresponding disk diffusion method. This susceptibility testing method is often used in Europe.
RESULTS AND DISCUSSION
Choices of disk diffusion testing reagents.
Preliminary tiamulin disk content studies of tiamulin used disk drug concentrations ranging from 5 to 90 μg. Each series of tests used four nonfastidious organisms, read by four technologists for a total of 10 replicates per strain and disk concentration. For tiamulin-susceptible strains of staphylococci, the mean zone diameters ranged from 22.4 to 31.7 mm (S. aureus) but nonsignificant increases in zones were achieved at disk drug concentrations greater than 30 μg (data not shown). The 30-μg tiamulin and erythromycin control disks produced similar zones of inhibition (mean diameters of 25.7 and 25.3 mm, respectively, for the control strain S. aureus ATCC 25923). The 30-μg concentration in disks or “tabs,” used previously in Europe, was adopted for subsequent experiments with disks produced commercially by BD Microbiology Systems.
Previous experiments in Denmark (1) using A. pleuropneumoniae strains (26 total) demonstrated average zone diameters of 20.3 mm at pH 8.0 and smaller zones at pH 7.2 to 7.4. The average MIC was 3.1 μg/ml (4 to 8 μg/ml at pH 7.4), and a susceptible breakpoint was suggested at ≤8 μg/ml, with a correlate zone diameter of ≥13 mm. However, these zones were based on technical or methodological details which did not conform to NCCLS documents for antimicrobial susceptibility tests with bacterial strains of animal origin, including (i) a tablet drug delivery system, (ii) semiconfluent growth inoculum (lighter than NCCLS-recommended confluent growth), and (iii) modified or elevated pH due to not using CO2 incubation, which favors fastidious-pathogen growth (5, 8). All of these modifications should result in reduced zone diameters of inhibition around 30-μg tiamulin disks used in the NCCLS procedure.
Susceptibility testing for tiamulin against nonfastidious pathogens.
Organisms listed in Table 1 were also tested with the 30-μg tiamulin disk (5). Two distinct populations of susceptible organisms were identified based on relative tiamulin potency: staphylococci, for which the MICs at which 90% of strains were inhibited (MIC90s) were 1 or 2 μg/ml and zone diameters were >20 mm, and P. multocida strains, for which MICs were 8 to 32 μg/ml and zone diameters were 10 to 21 mm. All other organisms listed in Table 1, such as enterococci and enteric and nonfermentative gram-negative bacilli, were resistant to tiamulin, with MICs being >32 μg/ml and median zone diameters being 6 to 12 mm.
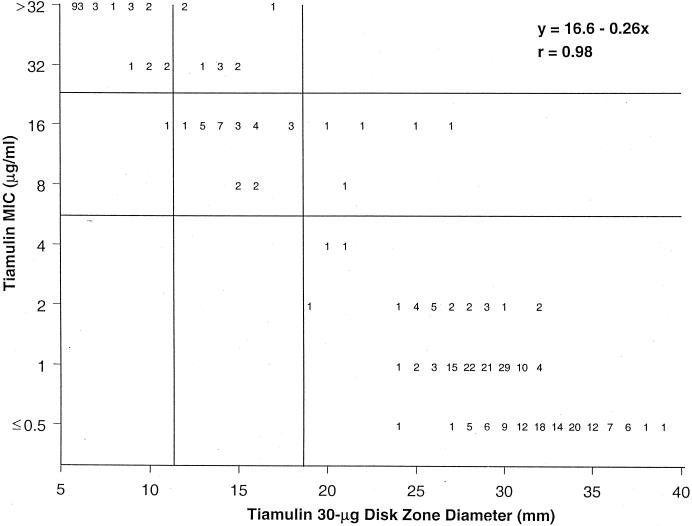
To accurately categorize these two groups of tiamulin-inhibited organisms, the staphylococci were declared susceptible (MIC breakpoint, ≤4 μg/ml) and P. multocida was categorized as moderately susceptible or intermediate (MIC range, 8 to 16 μg/ml). The correlate zone breakpoints were ≥19 mm for susceptibility and ≤11 mm for resistance (Fig. 1). These criteria produce an intermethod accuracy rate of 96.2%, and errors were as follows: very major (false susceptible) = 0.0%, major (false resistant) = 0.0%, and minor = 3.8%. All error rates were acceptable, and the results should indicate the need for possible dosage adjustments to address infections caused by strains for which tiamulin MICs are expected to be 8 or 16 μg/ml, such as P. multocida (see discussion of A. pleuropneumoniae below). The correlation coefficient was 0.98 (Fig. 1). Previously reported in vitro experience confirms our results (1, 2). This potency was similar to that noted for two fastidious species of Actinobacillus (Table 2).
FIG. 1.
Scattergram comparing reference tiamulin MICs to zone diameters around 30-μg tiamulin disks for 390 strains of nonfastidious, rapidly growing organisms. Horizontal lines show the proposed susceptibility (≤4 μg/ml) and resistance (≥32 μg/ml) MIC criteria. Vertical lines show the correlate interpretive zone diameters of ≥19 and ≤11 mm, respectively (96.2% intermethod agreement).
Susceptibility testing for tiamulin against five fastidious bacterial species.
Like the rapidly growing nonfastidious species tested against tiamulin by standardized NCCLS methods (5), the five fastidious species groups segregate into two levels of tiamulin susceptibility. The species that were tiamulin susceptible by the broth MIC results in VFM or HTM were E. rhusiopathiae, H. parasuis (HTM only), and the vast majority of S. suis isolates (MIC50, 1 to 2 μg/ml). The second, less-susceptible group included A. pleuropneumoniae, A. suis, and a very small number of S. suis isolates (MIC90, 16 μg/ml). The MICs for these species reported by the manufacturer and other investigators agree with these findings (12).
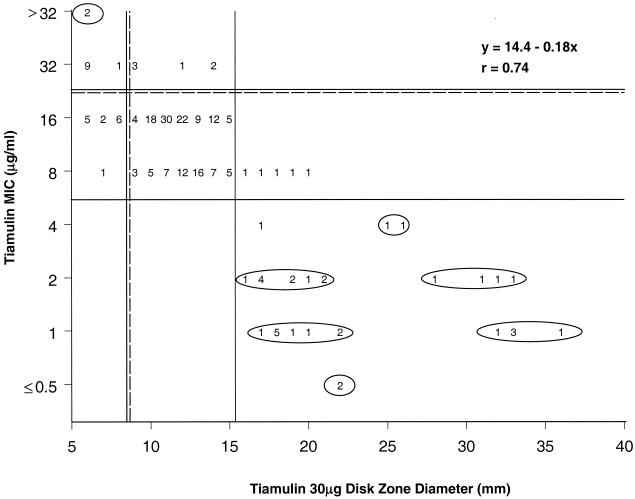
To establish susceptibility testing methods and remain consistent with the dose-related criteria suggested for nonfastidious species, the same MIC breakpoints (≤4 μg/ml for susceptibility; ≥32 μg/ml for resistance) were applied to these species. However, two distinct scattergram populations were also identified, where E. rhusiopathiae produced markedly larger zones around 30-μg tiamulin disks (slow grower) than S. suis strains; MICs for the two species in VFM (5) broth were the same (Fig. 2). Regardless of this phenomenon, a single set of interpretive criteria could easily be established for the disk diffusion method: ≥16 mm for susceptibility and ≤8 mm for resistance.
FIG. 2.
Scattergram comparing reference tiamulin MICs in VFM broth compared to zone diameters around 30-μg tiamulin disks when testing the fastidious veterinary pathogens: A. pleuropneumoniae, A. suis, E. rhusiopathiae, and S. suis (226 total strains). Horizontal and vertical solid lines indicate the proposed MIC breakpoints of ≤4 μg/ml (≥16 mm) as susceptible and ≥32 μg/ml as resistant for non-Actinobacillus species. Broken horizontal and vertical lines show the NCCLS-approved interpretive breakpoints for A. pleuropneumoniae (5). Circled numbers are non-Actinobacillus results.
Under these criteria, Actinobacillus spp. isolates, like P. multocida, would be categorized as moderately susceptible (intermediate) to tiamulin, requiring the appropriate (180 ppm in drinking water) dosing or intramuscular injections where indicated. However, we propose species-specific criteria for tiamulin as clinically indicated for A. pleuropneumoniae infections in swine: ≤16 μg/ml (≥9 mm) for susceptibility and ≥32 μg/ml (≤8 mm) for resistance. Intermethod absolute categorical accuracy was 90.5% for the 170 A. pleuropneumoniae strains and 90.1% for all 191 Actinobacillus strains tested. A similar modification (no intermediate range) could be applied to P. multocida among the nonfastidious species.
H. parasuis isolates were tested by comparing reference tiamulin MICs (in HTM) (5) and the zone diameters around 30-μg disks (on CMHA). Wide variations were noted in each test (data not shown), but the criteria for other fastidious species allowed acceptable accuracy between methods (7, 8) for testing tiamulin against H. parasuis. The small number of isolates tested (18 total; less than target numbers of ≥100 strains), the wide zone range variations at each MIC, and the use of HTM (not VFM) as the broth medium require further test development before definitive interpretive criteria can be recommended for this agent or any other.
In conclusion, tiamulin has a reputation as an effective veterinary-pathogen-specific antimicrobial that remains active against anaerobes, intestinal spirochetes, Actinobacillus spp., Pasteurella spp., and many common isolates of staphylococci and streptococci of animal and human origin (1-3, 12, 13). The proposed interpretive criteria (accepted by the NCCLS for A. pleuropneumoniae) for testing tiamulin against key indicated pathogens by NCCLS methods (5) will allow expanded testing of this agent and the accumulation of susceptibility statistics to monitor emerging resistance to the pleuromutilin class. These interpretive criteria are supplemented by recently published guidelines for quality control of tiamulin dilution and disk diffusion tests (5, 10). Care must be exercised to test the veterinary pathogens on appropriate media (5, 6) and to apply the species-specific breakpoint criteria and quality control procedures.
Acknowledgments
We express our appreciation to the following persons for their assistance in the completion of the study and the manuscript: T. Anderegg, J. Kirby, W. J. Howard, K. L. Meyer, J. Jones, D. J. Biedenbach, M. E. Erwin, M. L. Beach, and M. G. Stilwell.
The study was funded, in part, by an educational/research grant from Boehringer Ingelheim Vetmedica, Inc.
REFERENCES
- 1.Casals, J. B., R. Nielsen, and J. Szancer. 1990. Standardization of tiamulin for routine sensitivity of Actinobacillus (Haemophilus) pleuropneumoniae. Proc. Int. Pig Vet. Soc. 127:43. [Google Scholar]
- 2.Drews, J., A. Georgopoulos, G. Laber, et al. 1975. Antimicrobial activities of 81.723hfu, a new pleuromutilin derivative. Antimicrob. Agents Chemother 7:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitai, K., Y. Kashiwazaki, T. Adachi, and A. Kume. 1979. In vitro activity of 39 antimicrobial agents against Treponema [Brachyspira] hyodysenteriae. Antimicrob. Agents Chemother. 15:392-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall, S. A., R. N. Jones, A. Wanger, et al. 1996. Proposed MIC quality control guidelines for National Committee for Clinical Laboratory Standards susceptibility tests using seven veterinary antimicrobial agents: ceftiofur, enrofloxacin, florfenicol, penicillin G-novobiocin, pirlimycin, premafloxacin, and spectinomycin. J. Clin. Microbiol. 34:2027-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A. NCCLS, Wayne, Pa..
- 6.National Committee for Clinical Laboratory Standards. 1999. Development of in vitro susceptibility testing criteria and quality control parameters for veterinary antimicrobial agents. Approved guideline M37-A. NCCLS, Wayne, Pa.
- 7.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. NCCLS, Wayne, Pa.
- 8.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7. NCCLS, Wayne, Pa.
- 9.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters. Tentative guideline M23-A2. NCCLS, Wayne, Pa.
- 10.Pfaller, M. A., R. N. Jones, D. H. Walter, and The Quality Control Study Group. 2001. Proposed quality control guidelines for National Committee for Clinical Laboratory Standards susceptibility tests using the veterinary antimicrobial agent, tiamulin. Diagn. Microbiol. Infect. Dis. 40:67-70. [DOI] [PubMed] [Google Scholar]
- 11.Prescott, J., J. Baggot, and R. Walker (ed.). 2000. Antimicrobial therapy in veterinary medicine, 3rd ed., p. 257-262. Iowa State Press, Ames.
- 12.Sidoli, L., P. Barigazzi, P. Schianchi, et al. 1984. Minimum inhibitory concentrations of antibacterial agents against strains of Haemophilus [Actinobacillus] pleuropneumoniae from swine. Vet. Med. 79:703-705. [Google Scholar]
- 13.Werner, G., G. Laber, E. Schutze, et al. 1978. In vitro activity of tiamulin (81.723 hfu), a new pleuromutilin derivative against clinically significant anaerobes. J. Antibiot. 30:756-760. [DOI] [PubMed] [Google Scholar]