Summary
Mass movements of cytoplasm, known as cytoplasmic streaming, occur in some large eukaryotic cells. In Drosophila oocytes there are two forms of microtubule-based streaming. Slow, poorly ordered streaming occurs during stages 8-10A, while pattern formation determinants such as oskar mRNA are being localized and anchored at specific sites on the cortex. Then fast well-ordered streaming begins during stage 10B, just before nurse cell cytoplasm is dumped into the oocyte. We report that the plus-end-directed microtubule motor kinesin-1 is required for all streaming and is constitutively capable of driving fast streaming. Khc mutations that reduce the velocity of kinesin-1 transport in vitro blocked streaming yet still supported posterior localization of oskar mRNA, suggesting that streaming is not essential for the oskar localization mechanism. Inhibitory antibodies indicated that the minus-end-directed motor dynein is required to prevent premature fast streaming, suggesting that slow streaming is the product of a novel dynein-kinesin competition. As F-actin and some associated proteins are also required to prevent premature fast streaming, our observations support a model in which the actin cytoskeleton triggers the shift from slow to fast streaming by inhibiting dynein. This allows a cooperative self-amplifying loop of plus-end-directed organelle motion and parallel microtubule orientation that drives vigorous streaming currents and thorough mixing of oocyte and nurse-cell cytoplasm.
Keywords: Drosophila, Oocyte, Kinesin-1, Dynein, Streaming, Microtubule, Actin, Oskar
Introduction
Many mRNAs, proteins and organelles, whose synthesis occurs in discrete regions or compartments of cells, need to be dispersed throughout the cytoplasm for delocalized functions, while others need to be concentrated asymmetrically for localized functions. Cytoplasm is a gel-like fluid that allows small molecules to diffuse freely but restricts diffusion of large molecules and supramolecular complexes (Luby-Phelps, 2000). Thus, for both dispersal and for asymmetric localization of large complexes, cells have evolved machinery that actively transports cytoplasmic components that do not diffuse well (Saxton, 2001; Scholey et al., 2003; Shimmen and Yokota, 2004; Vale, 2003).
Intracellular arrays of cytoskeletal filaments (F-actin or microtubules) are required for most forms of active transport (Vale, 2003). The filaments, which have structural polarity, act as directional tracks for the transport of organelles or other cargoes by molecular motors; myosins that move on F-actin or kinesins and dyneins that move on microtubules. Many microtubule motors act as force-producing crosslinks with a mechanochemical, filament-binding ‘head’ at one end and a cargo-binding ‘tail’ at the other. Motors of the kinesin-1 subfamily (conventional kinesins) move cargoes towards microtubule plus-ends, which are usually distal to microtubule organizing centers (MTOCs), while cytoplasmic dynein moves cargoes toward minus-ends, which are usually at or near MTOCs. Thus, the positions of MTOCs and the paths of microtubules that project away from them dictate the directions and paths of microtubule-based cargo movements.
It is thought that kinesins and cytoplasmic dynein can attach to the same cargo, which raises questions about conflict between their opposing forces. Live imaging has shown that cargoes usually move alternately toward microtubule plus- and minus-ends, in a saltatory manner. Net transport is accomplished by a bias in favor of one direction (Mallik and Gross, 2004). Studies of melanosomes in Xenopus (Deacon et al., 2003), and lipid droplets, peroxisomes and mRNA particles in Drosophila (Gross et al., 2002b; Kural et al., 2005; Ling et al., 2004) suggest that minus- and plus-end microtubule motors strictly alternate rather than competing with one another in a tug-of-war. This raises the question of whether or not coordinated alternation of opposing motors is a universal feature of microtubule-based transport processes.
Drosophila oocytes provide a good system for investigating microtubule-dependent transport. Microtubule motors are important both for targeted localization of polarity determinant mRNAs, and for dispersal of components delivered to the oocyte anterior from adjoining nurse cells. During midoogenesis, bicoid (bcd), oskar (osk) and gurken (grk) mRNAs are localized in the oocyte to their respective anterior, posterior and dorsal positions in a kinesin-1- and dynein-dependent manner (Brendza et al., 2000a; Brendza et al., 2002; Duncan and Warrior, 2002; Januschke et al., 2002). Throughout that period of targeted localization, slow microtubule-dependent bulk streaming movements occur (Gutzeit, 1986b; Theurkauf et al., 1992). After polarity determinant localizations are well established, microtubule-based streaming becomes fast and well-ordered just before nurse cells non-selectively dump their contents into the anterior end of the oocyte (Gutzeit and Koppa, 1982). Coincident with the start of fast streaming, oocyte microtubules align to form bundles that lie parallel to the cortex (Theurkauf et al., 1992). Although kinesin-1, dynein and microtubules are important for these processes, how they contribute and how they relate to one another remains poorly understood.
To address issues about the mechanism of streaming and how it influences targeted localization and dispersal transport processes, we used time-lapse confocal microscopy to study the behavior of endosomes, determinant mRNAs and microtubules during slow and fast streaming. Tests of kinesin-1 and cytoplasmic dynein suggest a novel competitive relationship during slow streaming stages. Suppression of dynein activity allows a transition to robust, fast, plus-end movement by kinesin-1 that aligns microtubules into parallel arrays, which orders and amplifies plus-end cargo motion and thus fast streaming of surrounding cytoplasm. An allelic series of Khc mutations revealed that while posterior oskar mRNA localization did not require streaming, it did require some kinesin-1 activity, supporting the hypothesis that kinesin-1 can form physical links with oskar RNPs that contribute to posterior oskar localization by direct microtubule-based transport.
Materials and methods
Drosophila stocks and germline clones
To make germline clones, yw P{hs-FLP}; P{w+ FRT}42B P{OvoD1}55D/Cyo males were mated to: (1) w; P{w+, FRT}42B Khc17 Bc Elp px/CyO, (2) w; P{w+ FRT}42B b Khc23/CyO or (3) w; P{w+ FRT}42B c Khc27/CyO. Defects seen in egg chambers produced by germline clones of all three Khc alleles could be rescued by a wild-type Khc+ transgene (Saxton et al., 1991). Other strains acquired from the Bloomington Stock Center included: (1) w; P{w+ GAL4::VP16-nos.UTR}MVD1 P{w+, UASp-GFPS65c-α-tub84B}, Hu capu1, (2) cn1 bw1/Cyo, (3) l(2)DTS513 capuHK cn1 bw1/CyO and (4) spir1 cn1 bw1/Cyo l(2)DTS513. Wild-type controls all employed w1118 flies.
In situ hybridization and immunolabeling
For osk fluorescent in situ hybridization, flies were dissected in Robb's medium (in under 4 minutes), then fixed, rinsed and probed as described previously (Cha et al., 2002). Anti-α-tubulin staining was carried out as described previously (Brendza et al., 2002). Specimens were imaged with either a BioRad MRC600 scanning confocal or a PerkinElmer Ultraview spinning disk confocal fluorescence microscope.
Movies
For most endosome movies, 1 mg/ml Trypan Blue dye was injected into female fly abdomens and allowed to incubate for 2-7 hours. The dye, endocytosed with yolk by the oocyte, served as a bright fluorescence marker to make yolk granules more visible (B.-J.C., unpublished) (Danilchik and Denegre, 1991; Gutzeit and Arendt, 1994). Ovaries were dissected under halocarbon oil as described (Theurkauf, 1994b; Theurkauf and Hazelrigg, 1998). Antibody injections were made for a rabbit anti-Drosophila Khc (Cytoskeleton), a mouse monoclonal anti-Dhc (PIH4) from Tom Hays (McGrail and Hays, 1997), or a mouse monoclonal anti-DIC (74.1, Santa Cruz Biotechnology). All antibodies were dialyzed against PBS for at least 4 hours before injection at 2 mg/ml. Movies of Khc mutants were recorded with a BioRad MRC600 confocal, movies of antibody injections were made with a Leica TCS-SP confocal, and movies of GFP::α-tubulin were captured with a PerkinElmer Ultraview spinning disk confocal. Although acquisition rates varied between some sets of movies, all were compressed to 225× real time using QuickTime. Thus, 4 seconds of movie playback represents 15 minutes of real time in all videos.
Tracking
Digital organelle tracking was carried out with software created by Aaron Pilling (A. Pilling, PhD thesis, Indiana University, 2005). A grid was superimposed over the first frame of each movie. Grid line intersection coordinates were selected by a random choice generator, either restricted to the anterior half of stage 8-9 oocytes or throughout stage 10B-11 oocytes. The center of the endosome nearest each selected coordinate in the first frame was marked in succeeding frames with a cursor until it left the focal plane or until 95 frames had elapsed. During slow streaming in stage 8-9, images were collected every 15 seconds. During fast streaming, images were collected every 2.67 seconds. Endosomes that left the focal plane within five frames were not tracked. Ten endosomes were tracked per oocyte, and three to 13 oocytes were analyzed for each test.
To compare the effects of various experimental conditions on streaming velocities, we computed the distance between initial and final positions for each endosome, then divided by total time. This strategy produced slight underestimates of mean velocities, because the paths of some moving endosomes were curved. The advantage over the alternative approach, calculating distance from frame-frame position changes, was in suppressing the ‘noise’ of random saltation, which was substantial during slow streaming.
The amount of error caused by mis-marking of organelle centers and specimen drift was estimated by tracking non-saltating endosomes in dead oocytes. The calculated mean ‘tracking-error velocity’ in stages 8-9 was 1.1 nm/second±0.28 (s.e.m.) (n=20 endosomes). In stages 10B-11, that error increased to 6.4 nm/second±1.6 (n=20 endosomes) primarily because of the 5.6-fold decrease in time between images in the movies used to track fast streaming stages. Tracking-error velocity was subtracted from the raw velocity of each endosome tracked in live oocytes. Those with zero or negative corrected velocities were not included in calculation of the means for ‘moving organelles’. This was particularly important for analysis of stages 8-9, because many endosomes were stationary during slow streaming. After the error correction, endosomes with positive velocities were used to calculate means for each test, using SPSS 10.0.7 (SPSS). To determine peak velocities, means were calculated for the fastest 10% of endosomes tracked in each genotype.
Results
Kinesin-1 requirement for slow streaming
To address questions about microtubule motor contributions to the mechanism of ooplasmic streaming, an allelic series of recessive lethal Kinesin heavy chain (Khc) mutations was used to compromise kinesin-1 activity to varying degrees. Khc27 is a null allele (Q65-stop), whereas Khc23 and Khc17 are hypomorphic alleles caused by missense changes in the motor domain (S246F and E164K, respectively). In vitro motility assays with bacterially expressed motors from Khc23, Khc17 or wild-type constructs showed microtubule gliding velocities of 60, 140 or 230 nm/second, respectively (Brendza et al., 1999). The relative activities of the mutant kinesins in early development were determined by rendering the Khc alleles homozygous in germline stem cell clones of females (Brendza et al., 2000a; Chou et al., 1993; Golic and Lindquist, 1989) that were then mated to wild-type males (Table 1). Khc27 oocytes generated embryos that usually arrested during early cleavage or blastoderm stages (Brendza et al., 2000a). Khc23 oocytes generated offspring that arrested during later embryonic or larval stages, whereas most offspring from Khc17 oocytes completed development to adulthood.
Table 1.
Lethal phase analysis
| Germline genotype | Embryonic lethal | Larval/pupal lethal | Viable adults | n |
|---|---|---|---|---|
| Khc+ | 0 | 0 | 100% | 116 |
| Khc17 | 4% | 22% | 74% | 98 |
| Khc23 | 85% | 15% | 0 | 106 |
| Khc27 | 100% | 0 | 0 | 1078 |
The effects of the Khc mutant alleles on streaming were analyzed by time-lapse fluorescence microscopy of endosomes (yolk granules) in isolated egg chambers. Endosomes showed a wide range of sizes, with large organelles perhaps forming by fusion of smaller ones. Large endosomes seemed more prominent in Khc-null mutants, but variance between oocytes left a causal relationship uncertain. During stages 8 and 9 in wild-type oocytes, endosomes showed either short-range saltation with little net displacement, or moved in slow cytoplasmic streams that occurred primarily in the anterior half of the oocyte (Fig. 1A, arrow). Although the ‘streak projections’ of streaming shown in static figures are informative, movies provide a far deeper understanding of the real process and the effects of mutations (see Movies 1-13 in the supplementary material). To quantify velocities, randomly selected anterior endosomes were tracked (Fig. 1E). Those with velocities exceeding the mean for stationary endosomes in tracking-error controls (see Materials and methods) moved at a rate of 6.7±0.54 nm/second (n=49 organelles; n=5 oocytes). The peak velocity, determined as the mean for the fastest 10% of endosomes tracked, was 22±1.6 nm/second. In stage 8-9 Khc27 oocytes (n=5), no slow streaming currents were seen, but endosomes did show short-range saltation (Fig. 1D; see Movie 3 in the supplementary material) (Palacios and St Johnston, 2002). The mean velocity for anterior endosomes was ∼2.8-fold less than in wild type (Fig. 1E). Endosomes in hypomorphic Khc23 oocytes were similar, showing saltations, no slow streaming currents and ∼1.7-fold reduced mean velocity (Fig. 1C,E; see Movie 2 in the supplementary material). Some Khc17 oocytes produced minor slow streaming currents (Fig. 1B, arrow), but most endosomes showed only short-range saltation. The few streaming movements did not substantially contribute to the mean velocity of randomly selected endosomes, which was ∼2.4-fold less than wild type (Fig. 1E). Thus, even a relatively mild inhibition of Khc almost completely eliminated slow streaming currents, suggesting that slow streaming requires robust kinesin-1 function.
Fig. 1.
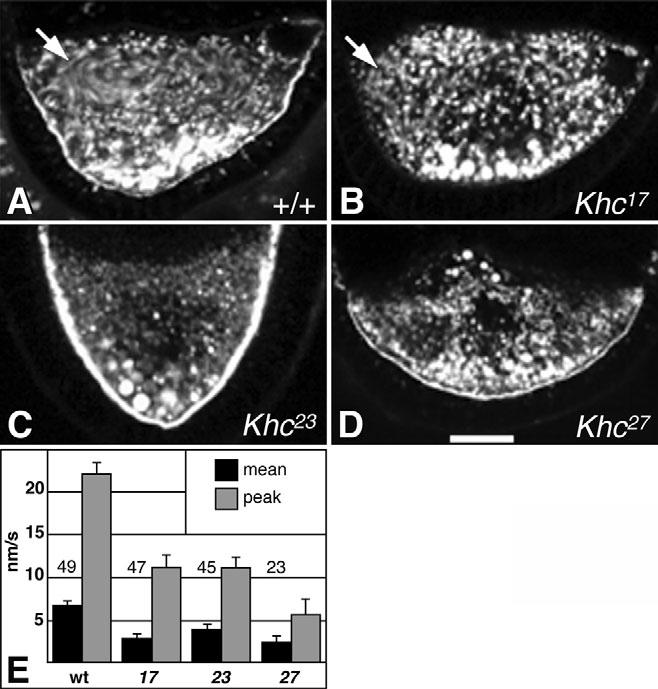
Effects of Khc mutations on endosome motion in oocytes during stage 9. (A-D and Movies 1-3 in the supplementary material) Fluorescent yolk endosomes were imaged in egg chambers during slow streaming stages. Each panel is a projection of 10 images from one focal plane acquired at 15-second intervals. Moving endosomes appear as elongated streaks, while non-moving endosomes appear as circular spots. Genotypes are noted in each panel. Arrows in A and B indicate areas that had streaming movements (see Movie 1 in the supplementary material). (E) Overall mean and peak endosome velocities are shown for each genotype. Numbers in or above bars show sample sizes. Peak velocities were calculated as means of the fastest 10% of endosomes of each genotype. Scale bar: 25 μm.
Determinant mRNA localization in the absence of slow streaming
Slow streaming currents may be important for random movements of determinant mRNA particles that facilitate contact with anchorage sites in specific regions of the oocyte cortex (Cha et al., 2002; Glotzer et al., 1997). Consistent with this, the null Khc27 allele, which eliminates slow streaming currents, prevents oskar mRNA accumulation at the posterior pole and hinders gurken mRNA accumulation at the anterodorsal corner (Brendza et al., 2000a; Brendza et al., 2002; Ephrussi et al., 1991; Kim-Ha et al., 1991; Neuman-Silberberg and Schupbach, 1993). A recent model suggests that, by walking on microtubules that have minus-ends at the cortex and plus-ends away from the cortex, kinesin-1 drives oskar mRNPs towards the center of the oocyte. Then random movements such as diffusion and/or slow streaming deliver those mRNPs to cortical anchorage sites at the posterior pole where microtubule density is lowest (Cha et al., 2002). To address the question of whether or not slow streaming is required for mRNP localization, we used fluorescence in situ hybridization to study effects of the hypomorphic Khc alleles (Khc23 and Khc17) on gurken and oskar mRNA localization. Despite the absence of slow streaming currents in the mutant oocytes, gurken localization often appeared normal (14 out of 24 Khc23 and seven out of seven Khc17 oocytes; see Fig. S1 in the supplementary material). Likewise, oskar mRNA localized to the posterior pole in all hypomorphic Khc oocytes examined (Fig. 2B,C; see Fig. S2 in the supplementary material), although its concentration may have been somewhat reduced. Thus, slow streaming is not an essential element of the gurken or oskar mRNA localization mechanisms.
Fig. 2.
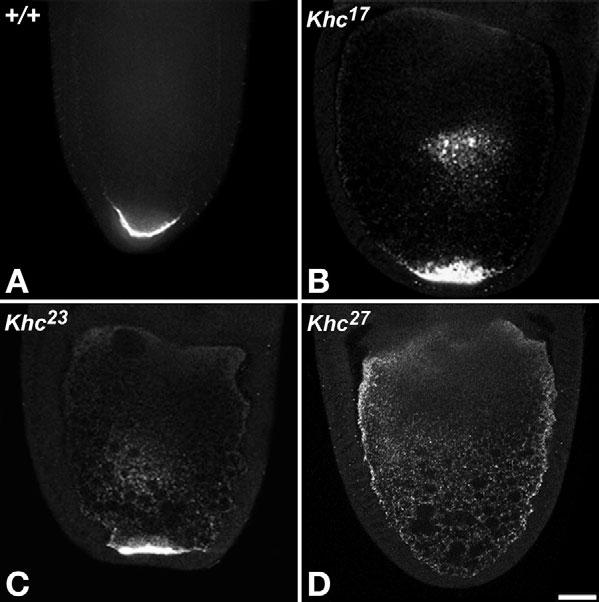
Hypomorphic Khc alleles that block slow streaming did not block oskar mRNA localization. In situ hybridization with fluorescent oskar RNA probes in stage 10A-10B shows localization of oskar mRNA in oocytes with the indicated genotypes. (A) Wild type. (B,C) Some oskar accumulated and persisted at the centers of the hypomorphic mutant oocytes and concentrated strongly at the posterior (see also Fig. S2 in the supplementary material). (D) In Khc-null oocytes, oskar did not accumulate at either the center or posterior, but there was an elevated concentration around the cortex. Scale bar: 25 μm.
Partial kinesin-1 inhibition can eliminate fast streaming
During stage 10B, shortly before the massive influx of cytoplasm from nurse-cells, oocytes switch from slow streaming, mainly at the anterior, to well-ordered fast streaming throughout the oocyte (Gutzeit and Koppa, 1982). To study the contributions of kinesin-1 to fast streaming, endosome movements in stage 10B-11 oocytes were recorded by time-lapse microscopy and analyzed by digital tracking (Fig. 3; Movies 4-6 in the supplementary material). In wild type, randomly selected endosomes with net velocities above tracking error had a mean velocity of 120±6.4 nm/second (n=126 organelles; n=13 oocytes) and a peak velocity of 360±9.0 nm/second (Fig. 3A,E). In Khc27 oocytes, no fast streaming currents were seen (Fig. 3D) (Palacios and St Johnston, 2002). Rather, endosomes underwent short-range saltation and little net displacement (see Movie 6 in the supplementary material), moving ∼32-fold slower than wild type (Fig. 3E). Interestingly, yolk endosomes were concentrated towards the posterior, leaving a clear zone at the anterior (Fig. 3D; eight out of nine oocytes). The stratified ooplasm implies a failure of streaming-driven mixing of yolk-free nurse cell contents with yolk-containing ooplasm. It also shows that the force from dumping itself did not generate fast streaming currents, in agreement with microtubule depolymerization tests by others (Gutzeit, 1986b; Koch and Spitzer, 1983; Theurkauf, 1994a).
Fig. 3.
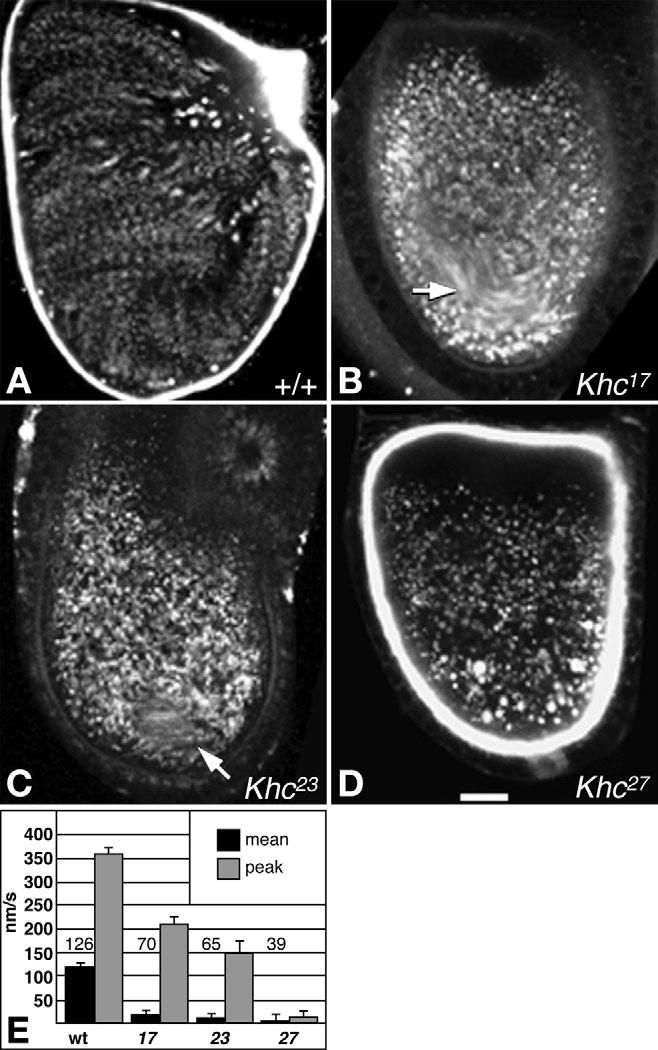
Effects of Khc mutations on endosome motion in oocytes during stages 10B-11. (A-D and Movies 4-6 in the supplementary material) Fluorescent yolk endosomes were imaged in isolated egg chambers during fast streaming stages. Each panel is a projection of 10 images from one focal plane acquired over 150 seconds. Fast-moving endosomes appear as strings of dots, while slower endosomes appear as blurred streaks. Non-moving endosomes appear as circular spots. Genotypes are indicated in each panel. Arrows in B,C indicate areas that had streaming movements. In C, the radial streaks at the upper right corner are from movement of debris outside the egg chamber. (E) Overall mean and peak endosome velocities are shown for each genotype. Numbers in or above bars show sample sizes for overall means. Peak velocities were calculated as means of the fastest 10% of endosomes for each genotype. Scale bar: 25 μm.
Partial inhibition of kinesin-1 by the two hypomorphic Khc alleles blocked or severely reduced fast streaming. In Khc23 oocytes, saltation and some short, individual endosome displacements occurred, but concerted fast currents were rare, occurring in a small region of only one out of the nine oocytes observed (Fig. 3C arrow). Randomly selected Khc23 endosomes moved at a mean velocity ∼11-fold less than wild type (Fig. 3E). In Khc17 oocytes, similar saltation and short, individual displacements occurred, but concerted fast streaming currents were more common (four out of 15 oocytes). In two cases, weak streaming occurred throughout the oocyte. In the other two cases, streaming was restricted to a smaller region (Fig. 3B arrow; see Movie 5 in the supplementary material). Randomly selected endosomes moved at rates ∼7-fold less than wild type (Fig. 3E). These results confirm that kinesin-1 is the primary motor for fast streaming (Palacios and St Johnston, 2002). Little evidence of endosome stratification was seen in the hypomorphic mutant oocytes, suggesting that even short-range individual organelle displacements driven by the mutant Khc motors accomplished some mixing.
Dynein and the actin cytoskeleton inhibit kinesin-1-driven fast streaming
Cytoplasmic dynein is the primary motor for minus-end-directed microtubule-based transport in metazoan animals (Vale, 2003). Because of its fundamental importance, a severe loss of dynein function is cell lethal and prevents oocyte development (McGrail and Hays, 1997). Based on tests with mild, non-lethal dynein alleles, it has been suggested that dynein contributes force to help drive fast streaming (Palacios and St Johnston, 2002). To test the effects of more severe disruptions of dynein function, inhibitory antibodies specific for dynein intermediate chain (DIC), dynein heavy chain (Dhc) or non-specific IgG were injected into oocytes during time-lapse imaging of endosome movement (Fig. 4; see Movies 7 and 8 in the supplementary material). Anti-DIC injected into stage 10B-11 oocytes undergoing fast streaming had no clear effect (n=15). Surprisingly, injection of anti-DIC antibody into stage 8-9 oocytes induced a conversion from slow streaming to faster, more ordered streaming, particularly in the anterior regions (34 out of 41 oocytes; Fig. 4A,B; see Movie 7 in the supplementary material). Similar premature fast streaming activity was also triggered by anti-Dhc injections (16 out of 20 oocytes; Fig. 4C,D; see Movie 8 in the supplementary material). Control IgG injections had no effect (n=9; Fig. 4E). Furthermore, injection of anti-DIC into oocytes null for Khc did not induce fast streaming. Rather, endosomes showed only saltatory movement (n=15; Fig. 4F). Therefore, the premature fast streaming caused by dynein inhibition in wild-type oocytes was due to early induction of the normal kinesin-1-dependent fast streaming mechanism. These results suggest that the minus-end-directed motor cytoplasmic dynein represses premature fast streaming during mid-oogenesis, perhaps by antagonizing plus-end-directed forces produced by kinesin-1.
Fig. 4.
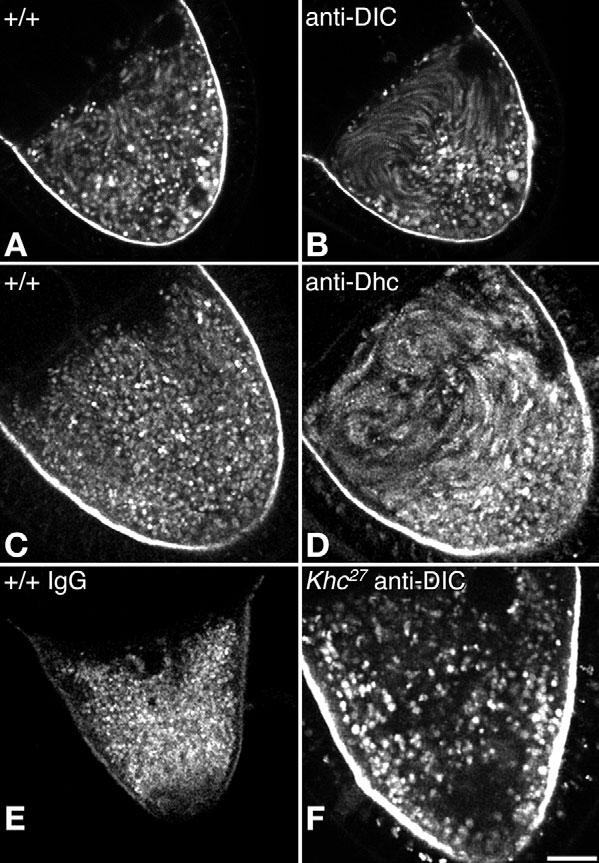
Effects of anti-dynein injections on endosome movement in oocytes during stages 9-10A. Each panel is a projection of eight images from one focal plane acquired at 20-second intervals. Moving endosomes appear as elongated streaks while non-moving endosomes appear as circular spots. (A-D) Wild-type oocytes are shown before injection (A,C) and after injection with antibodies specific for (B) dynein intermediate chain (anti-DIC) or (D) dynein heavy chain (anti-Dhc). (E) A wild-type oocyte after injection with nonspecific IgG. (F) A Khc-null oocyte after injection with anti-DIC. Better views of the effects of antibody injections are provided in Movies 7 and 8 in the supplementary material. Scale bar: 25 μm.
The actin cytoskeleton also has an important role in repressing premature fast streaming. Fast streaming has been seen as early as stage 4 when F-actin is disrupted with cytochalasin D (Emmons et al., 1995) or when the actin regulators Cappuccino, Spire or Chickadee (profilin) are compromised by genetic mutations (Emmons et al., 1995; Magie et al., 1999; Manseau et al., 1996; Otto et al., 2000; Theurkauf, 1994b; Verheyen and Cooley, 1994; Wellington et al., 1999). To determine if such premature fast streaming is dependent on kinesin-1, inhibitory anti-Khc antibody was injected into either wild type, capu or spir mutant oocytes during time-lapse imaging. In wild-type stage 10B-11 oocytes, control IgG injection had little effect (Fig. 5A; n=8 oocytes), whereas injection of anti-Khc slowed or stopped fast streaming in 15 of 17 oocytes (Fig. 5B). Thus, the anti-Khc was effective at blocking kinesin-1 activity. When injected into stage 9-10A oocytes, Khc antibody hindered premature fast streaming in eight out of 12 capu mutants (Fig. 5C,D) and nine out of 11 spir mutants (Fig. 5E,F; see Movie 11 in the supplementary material). Control IgG and anti-DIC injection did not detectably affect streaming in either mutant background (n=12 and n=7, respectively). These results indicate that the premature fast streaming in capu and spir mutants reflects induction of the normal kinesin-1-driven process rather than some novel streaming mechanism. Combined with the premature fast streaming induced by dynein inhibition, this suggests that although kinesin-1 has the capability to drive well-ordered fast streaming before stage 10B, it is repressed by a mechanism that requires a normal actin cytoskeleton and active cytoplasmic dynein.
Fig. 5.
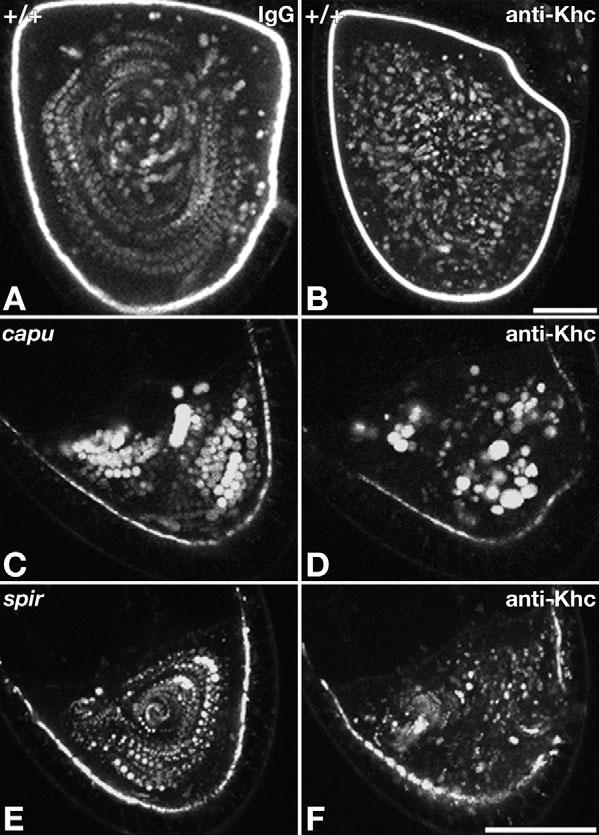
Anti-Khc injections blocked premature fast streaming in capu and spir mutants. Each panel is a projection of eight images from one focal plane acquired at 20-second intervals (see Movies 9-11 in the supplementary material). (A,B) Stage 11 wild-type oocytes injected with control antibody (A) or anti-Khc (B) show that antibody inhibition of kinesin-1 stopped normal fast streaming (see also Movies 9 and 10 in the supplementary material). (C,D) A capu1/capuHK oocyte before (C) and after (D) anti-Khc injection. (E,F) A spir1/spir1 oocyte before (E) and after (F) anti-Khc injection (Movie 11 in the supplementary material). Scale bars: in B, 50 μm for A,B; in D, 50 μm for C-F.
Disordered microtubules in slow streaming versus ordered microtubules in fast streaming
Based on staining of fixed egg chambers (e.g. Fig. 6C), it has been suggested that formation of subcortical microtubule bundles that lie parallel to the oocyte surface is an important element of the fast streaming mechanism (Theurkauf et al., 1992), and that bundling may trigger the conversion from slow to fast streaming (Manseau et al., 1996). To investigate the relationship of microtubule organization to the slow/fast streaming modes, tubulin antibodies and GFP::α-tubulin were imaged by confocal fluorescence microscopy. During slow streaming (stages 8-10A), microtubules were more abundant at the oocyte anterior than at the posterior, but they had the appearance of a random network lacking detectable order (Fig. 6A,B). This was seen most clearly in time-lapse movies focused near the cortex, where image contrast was highest. Surprisingly, microtubules near the anterior were in a constant state of motion and dynamic reorientation (see Movie 12 in the supplementary material). This adds to arguments that, although minus-ends are embedded in the oocyte cortex (Theurkauf et al., 1992), most microtubules are not otherwise well-ordered during stages 8-10A (Cha et al., 2002). We saw no evidence supporting the hypothesis that plus ends are uniformly directed towards the posterior pole.
Fig. 6.
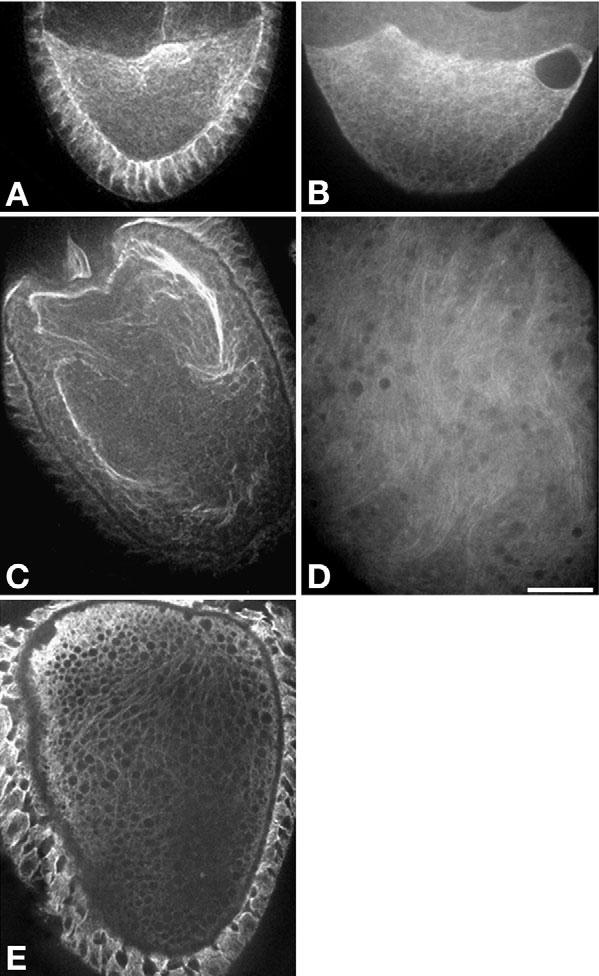
Oocyte microtubule behavior during slow and fast streaming stages. Spinning disk confocal images of (A) a fixed stage 9 oocyte (slow streaming stage) with fluorescence from α-tubulin antibody staining and of (B) a live stage 9 oocyte with fluorescence from transgenic GFP::α-tubulin. Time-lapse movies of GFP fluorescence show that randomly oriented microtubules underwent dynamic churning motions (which can only be seen in Movie 12 in the supplementary material). (C) A fixed stage 10B oocyte (fast streaming stage) with fluorescence from α-tubulin antibody staining shows large microtubule bundles. (D) A live stage 10B fast streaming oocyte with fluorescence from GFP::α-tubulin. Time-lapse movies of the GFP show that microtubules joined in loose, parallel arrays rather than tight bundles. The arrays were dynamic transient structures that aligned and bent with streaming currents (which can only be seen in Movie 13 in the supplementary material). (E) A fixed stage 10B Khc17 oocyte stained with α-tubulin antibody that shows some aligned fine filamentous structures, but no large microtubule bundles. Scale bar: 25 μm.
During fast streaming in stages 10B-11, GFP-microtubules were more difficult to image, but movies revealed a higher degree of order. Close to the cortex, it was evident that microtubules formed loose parallel arrays that often split and bent sinuously, while remaining oriented along the changing paths of rapid cytoplasmic streaming currents (see Movie 13 in the supplementary material). This striking behavior during fast streaming suggests that the large microtubule bundles observed after fixation and immunostaining (Fig. 6C) misrepresent the true nature of loose and dynamic parallel microtubule arrays.
To determine if extensive parallel microtubule arrays are a prerequisite for fast streaming, the influence of Khc17, which supported fast streaming in some oocytes but not others, on microtubules was studied in stage 10B-11 oocytes. GFP::α-tubulin movies of Khc17 oocytes showed no evidence of large ordered arrays (n=7). However, small parallel arrays might have been obscured by low image contrast. To take advantage of the bundling effect of fixation, Khc17 oocytes were fixed and stained with anti-tubulin (Fig. 6E). No evidence of large microtubule bundles was seen (n=8). As fast streaming currents were seen in 27% of Khc17 oocytes, if parallel microtubule arrays were a regulatory prerequisite, some bundles should have been seen in the fixed oocytes. This suggests that ordered microtubule arrays are not required to initiate fast streaming. It also suggests that a threshold amount of kinesin-1 activity greater than that provided by Khc17 is required for strong array formation. Perhaps robust fast streaming and parallel bundling of microtubules comprise a cooperative loop in which unopposed kinesin-1-driven streaming encourages parallel orientation, and parallel orientation encourages more robust streaming currents.
Discussion
To address questions about microtubule-based cytoplasmic streaming in Drosophila oocytes, function disruption approaches were combined with fixed and time-lapse fluorescence microscopy. Our results confirm that plus-end-directed kinesin-1 is the primary motor for both slow and fast streaming (Palacios and St Johnston, 2002), and, furthermore, that it is constitutively capable of driving fast streaming. The minus-end-directed motor cytoplasmic dynein does not contribute force for fast streaming, rather dynein and a normally regulated actin cytoskeleton impede the fast streaming activity of kinesin-1, allowing only slow streaming currents prior to stage 10B.
Ooplasmic streaming and development
It is reasonable to assume that the purpose of active but random transport processes like streaming is to facilitate the dispersal of cytoplasmic components that do not diffuse fast enough to support cellular and developmental demands. However, it could also be important for asymmetric localization processes by facilitating encounters of cytoplasmic components with localized anchors (Cheeks et al., 2004; Glotzer et al., 1997). More specific insights into how microtubule-based streaming contributes to particular processes have been elusive, in part because the only means to prevent it was to eliminate microtubules, which are needed for many fundamental cellular processes. Identification of kinesin-1 as the motor for streaming in Drosophila (this report) (Palacios and St Johnston, 2002) provides the opportunity for more focused studies, because it has a narrower range of functions and is not essential for early oocyte development (Brendza et al., 2000b).
Our Khc allelic series allowed investigation of the significance of nurse cell/ooplasm mixing. Khc-null oocytes, with no streaming, usually showed yolk stratification as evidence of mixing failure. Embryos developing from those oocytes arrested in early stages, suggesting that mixing may be important for subsequent development. However, hypomorphic Khc17 oocytes, which supported weak fast streaming in only one-third of oocytes, allowed three-fourths of the derived embryos to develop to adulthood (Table 1). Yolk stratification was not seen in Khc17 oocytes, suggesting that some mixing can occur without ordered streaming. Although these observations are consistent with the hypothesis that vigorous ooplasmic mixing helps optimize development, it is likely that fast streaming is not absolutely essential.
The Khc allelic series also allowed exploration of a role for slow ooplasmic streaming in determinant mRNA localization. The null allele Khc27 prevented streaming, it blocked oskar mRNA accumulation at the posterior pole and it blocked gurken mRNA localization to the anterodorsal corner (Brendza et al., 2002; Cha et al., 2002; Duncan and Warrior, 2002; Januschke et al., 2002). However, the hypomorphic alleles Khc17 and Khc23, which prevented most slow streaming, supported both oskar and gurken localization (Figs 1, 2; see Figs S1, S2 in the supplementary material). Thus, although localization of both determinants requires Khc, it does not require slow streaming.
Kinesin-1 and the mechanism of oskar mRNA localization
It has been suggested that posterior oskar localization during stages 7-10a proceeds via two phases (Cha et al., 2002). First, oskar RNPs are driven by kinesin-1 away from microtubule minus ends at the anterior and lateral cortex, which leads to a transient concentration of oskar in the central region of the oocyte. Then diffusion or other random forces, coupled with a dearth of minus ends at the posterior cortex, facilitates encounters of oskar RNPs with posterior anchors. Our tests of Khc17 and Khc23, which slow the ATPase activity and velocity of Khc in vitro, showed a delay in the central accumulation of oskar, consistent with slowed kinesin-1-driven transport away from the anterolateral cortex. Strikingly, Khc17 and Khc23 allow that central accumulation to persist through later stages, as if the shift to posterior anchors is also slowed (see Fig. S2 in the supplementary material). This correlation between slowed motor mechanochemistry and slowed oskar localization supports the hypothesis that kinesin-1 links to and transports oskar RNPs in both phases of localization.
If microtubules are poorly ordered during oskar localization, as suggested by our GFP-tubulin imaging and by previous studies of fixed oocytes (Brendza et al., 2002; Cha et al., 2002; Theurkauf et al., 1992), how could kinesin-1 accomplish such directed posterior transport? There may be a special subset of microtubules, with plus-ends oriented directly toward the posterior pole, that are difficult to distinguish amongst a mass of randomly oriented microtubules. However, given that the period of oskar localization spans at least 10 hours, and that the distance from the oocyte center to the posterior pole is only 25-40 μm, such perfectly oriented transport tracks should not be necessary. With microtubule minus ends most abundant at the anterior cortex and least abundant at the posterior cortex, plus ends should be somewhat biased toward the posterior. If kinesin-1 binds an oskar RNP and transports it to a plus end, then binds a neighboring microtubule and runs to its plus end, and so forth, it would accomplish a biased random walk away from the anterolateral cortex that would concentrate oskar RNPs near posterior anchors. This highlights a central question about the mechanism of localization. What is the degree of directional bias for oskar RNP transport? Advances in osk RNP imaging that allow single particle tracking will be needed to obtain clear answers to that question.
Kinesin-1/dynein competition in ooplasmic streaming
Regarding the mechanism of streaming, we suggest a model in which kinesin-1 drives plus-end-directed motion of cargoes that act as impellers, exerting force on ooplasm that surrounds them (Fig. 7). Concerted movement of multiple impellers along neighboring microtubules that are oriented in the same general direction creates streams of ooplasm. Prior to stage 10B, small streams occur, but are slow and not well-ordered because dynein resists both plus-end-directed transport and parallel ordering of microtubules. This resistance may be accomplished via: (1) a tug-of-war between opposing motors co-attached to individual impellers (Theurkauf, 1994b); (2) by movement of different impellers in opposite directions, imparting conflicting forces on cytoplasm; or (3) competition by dynein and kinesin for the same binding site on microtubules (Mizuno et al., 2004). Regardless of how dynein interferes with kinesin-1, just before nurse cell cytoplasm is dumped into the oocyte, dynein is suppressed. This allows kinesin-1 to generate fast plus-end-directed impeller transport that sweeps microtubules into parallel arrays that then enhance more robust currents that enhance larger arrays, and so forth, in a self-amplifying loop.
Fig. 7.
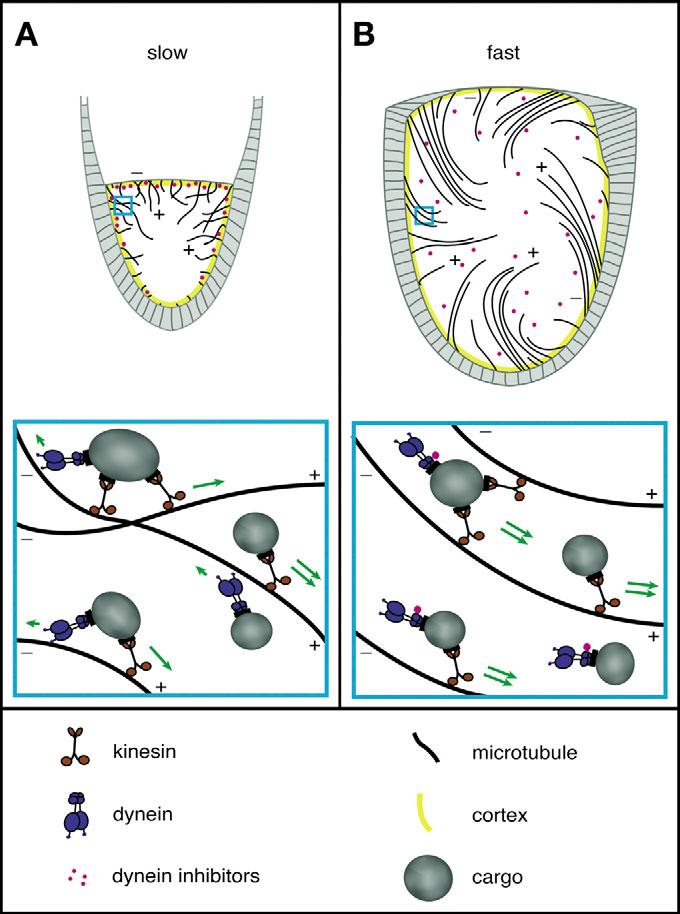
A speculative model for slow and fast streaming in Drosophila oocytes. The lower panels represent expanded views of the regions enclosed by blue boxes in the upper panels. The underlying premise is that unidirectional transport of organelles along microtubules can impel surrounding cytoplasm to stream. (A) Before stage 10B, competition between dynein and kinesin-1 suppresses concerted plus-end organelle transport and prevents parallel ordering of microtubules, allowing only slow short-range currents. (B) During stage 10B, a dynein inhibitory signal is released from the actin-rich cortex. This allows concerted kinesin-driven organelle transport to impel strong plus-end-directed currents and sweep microtubules into parallel, but flexible, dynamic arrays.
Our finding that dynein inhibition enhances a kinesin-1-driven transport process, to our knowledge, provides the first direct indication of a competitive relationship between opposing microtubule motors. Other studies have produced convincing evidence of alternating coordination between dynein and plus-end-directed motors in a number of processes, including transport of Drosophila embryo lipid droplets (Gross et al., 2002b), Drosophila cultured cell RNPs and peroxisomes (Kural et al., 2005; Ling et al., 2004), Drosophila axonal mitochondria (A. Pilling, PhD thesis, Indiana University, 2005), and Xenopus pigment granules (Deacon et al., 2003). In those processes, inhibition of one motor does not enhance transport in the opposite direction. In fact kinesin-1 inhibition inhibits not only plus-end transport but also dynein-driven minus-end transport (Brady et al., 1990; Kural et al., 2005) (A. Pilling, PhD thesis, Indiana University, 2005). Furthermore, dynein depletion can inhibit both directions of peroxisome transport (Kural et al., 2005), confirming that kinesin-1 and dynein each can have positive influences on the other. Our observation of competition between dynein and kinesin-1 suggests that alternating coordination and positive interactions between microtubule motors are not a uniform rule, and that some processes have evolved to take advantage of motor competition.
If slow streaming is a product of kinesin-dynein competition, why does Khc inhibition arrest all streaming (Movies 10 and 11 in the supplementary material), rather than freeing dynein to drive reverse streaming? One possibility is that although forces from impeller-bound dynein can resist kinesin-1 and confound parallel microtubule ordering, it is not sufficiently processive to generate minus-end-directed streaming currents. A second possibility is that Khc inhibition blocks minus-end as well as plus-end-directed streaming forces, similar to the processes noted above in which dynein transport activity is dependent on Khc (Brendza et al., 2002; Kural et al., 2005; Martin et al., 1999) (A. Pilling, PhD thesis, Indiana University, 2005).
Control of kinesin-1-based fast streaming by the actin cytoskeleton
The observation that actin cytoskeleton depolymerization or mutation of certain actin-interacting proteins can induce premature kinesin-1-driven fast streaming is particularly interesting. Actin filaments are most abundant in the cortex and ring canals of the oocyte and nurse cells (Gutzeit, 1986a; Warn et al., 1985), but filaments probably also traverse the internal cytoplasm. An intact actin cytoskeleton could physically assist dynein in resisting kinesin-based plus-end-directed transport during slow streaming, either passively by increasing viscosity or actively by generating antagonistic forces. The active force idea is supported by reports that myosin V can alter the balance between alternating dynein and kinesin-2-driven runs of melanosomes in Xenopus (Gross et al., 2002a). Drosophila myosin V inhibition tests have not yet been reported, but a disordered cortical actin cytoskeleton in Moesin mutant oocytes did not trigger premature fast streaming (Polesello et al., 2002), suggesting that well-ordered actin-based forces may not be important for the streaming control mechanism. An alternative to such physical resistance is that dynein inhibitory factors are sequestered by F-actin prior to stage 10B. Then, just before dumping, those factors are released, dynein is inhibited, and kinesin-1 is freed to drive fast streaming (Fig. 7).
Recently, several other factors have been identified that are required for prevention of premature fast streaming. Mutations in Maelstrom (Mael), Orb and Spindle-E (Spn-E) allow premature fast streaming and parallel microtubule arrays during stages 8-10A (Clegg et al., 1997; Martin et al., 2003). Orb, a CPEB homolog, is required for osk translation (Chang et al., 1999), spn-E is an RNA helicase (Gillespie and Berg, 1995), and Mael is a modifier of Vasa (Findley et al., 2003), which is another RNA helicase (Hay et al., 1988; Liang et al., 1994). Perhaps these proteins control expression of actin regulators or other factors needed to prevent premature activation of a dynein inhibitory signal. Future work aimed at identifying the regulatory mechanisms that control kinesin in oocytes should be an important focus in understanding the slow-fast streaming transition and also for the broader issue of how the functions of the actin and microtubule cytoskeletons are integrated.
Supplementary Material
Acknowledgments
We thank Aaron Pilling for guidance with tracking, Joseph Duffy for assistance with in situ hybridization, Curt Lively for statistical advice, and Tom Hays for providing Dhc antibodies. We also thank Anne Ephrussi, Volodya Gelfand, David Ish-Horowitz, Steve Gross, Rahul Warrior and Michael Welte for their creative insights. B.-J.C. was supported by a postdoctoral fellowship from the Harold Whitworth Pierce Charitable Trust. This work was supported by NIH R01-GM46295 (W.M.S.), NIH RO1-HD049116 (W.E.T.) and ACS Research Scholar Grant RGS CSM-101560 (W.E.T.).
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/132/16/3743/DC1
References
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza KM, Rose DJ, Gilbert SP, Saxton WM. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J. Biol. Chem. 1999;274:31506–31514. doi: 10.1074/jbc.274.44.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000a;289:2120–2122. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Sheehan KB, Turner FR, Saxton WM. Clonal tests of conventional kinesin function during cell proliferation and differentiation. Mol. Biol. Cell. 2000b;11:1329–1343. doi: 10.1091/mbc.11.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Serbus LR, Saxton WM, Duffy JB. Posterior localization of dynein and dorsal-ventral axis formation depend on kinesin in Drosophila oocytes. Curr. Biol. 2002;12:1541–1545. doi: 10.1016/s0960-9822(02)01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha BJ, Serbus LR, Koppetsch BS, Theurkauf WE. Kinesin I-dependent cortical exclusion restricts pole plasm to the oocyte posterior. Nat. Cell Biol. 2002;4:592–598. doi: 10.1038/ncb832. [DOI] [PubMed] [Google Scholar]
- Chang JS, Tan L, Schedl P. The Drosophila CPEB homolog, orb, is required for oskar protein expression in oocytes. Dev. Biol. 1999;215:91–106. doi: 10.1006/dbio.1999.9444. [DOI] [PubMed] [Google Scholar]
- Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr. Biol. 2004;14:851–862. doi: 10.1016/j.cub.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Chou TB, Noll E, Perrimon N. Autosomal P{ovoD1} dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development. 1993;119:1359–1369. doi: 10.1242/dev.119.4.1359. [DOI] [PubMed] [Google Scholar]
- Clegg NJ, Frost DM, Larkin MK, Subrahmanyan L, Bryant Z, Ruohola-Baker H. maelstrom is required for an early step in the establishment of Drosophila oocyte polarity: posterior localization of grk mRNA. Development. 1997;124:4661–4671. doi: 10.1242/dev.124.22.4661. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Denegre JM. Deep cytoplasmic rearrangements during early development in Xenopus laevis. Development. 1991;111:845–856. doi: 10.1242/dev.111.4.845. [DOI] [PubMed] [Google Scholar]
- Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 2003;160:297–301. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JE, Warrior R. The cytoplasmic dynein and kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr. Biol. 2002;12:1982–1991. doi: 10.1016/s0960-9822(02)01303-9. [DOI] [PubMed] [Google Scholar]
- Emmons S, Phan H, Calley J, Chen W, James B, Manseau L. Cappuccino, a Drosophila maternal effect gene required for polarity of the egg and embryo, is related to the vertebrate limb deformity locus. Genes Dev. 1995;9:2482–2494. doi: 10.1101/gad.9.20.2482. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- Gillespie DE, Berg CA. Homeless is required for RNA localization in Drosophila oogenesis and encodes a new member of the DEH family of RNA-dependent ATPases. Genes Dev. 1995;9:2495–2508. doi: 10.1101/gad.9.20.2495. [DOI] [PubMed] [Google Scholar]
- Glotzer JB, Saffrich R, Glotzer M, Ephrussi A. Cytoplasmic flows localize injected oskar RNA in Drosophila oocytes. Curr. Biol. 1997;7:326–337. doi: 10.1016/s0960-9822(06)00156-4. [DOI] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 2002a;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP, Welte MA, Block SM, Wieschaus EF. Coordination of opposite-polarity microtubule motors. J. Cell Biol. 2002b;156:715–724. doi: 10.1083/jcb.200109047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit HO. The role of microfilaments in cytoplasmic streaming in Drosophila follicles. J. Cell Sci. 1986a;80:159–169. doi: 10.1242/jcs.80.1.159. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO. The role of microtubules in the differentiation of ovarian follicles during vitellogenesis in Drosophila. Wilhelm Roux's Arch. Dev. Biol. 1986b;195:173–181. doi: 10.1007/BF02439435. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO, Koppa R. Time-lapse film analysis of cytoplasmic streaming during late oogenesis of Drosophila. J. Embryol. Exp. Morphol. 1982;67:101–111. [Google Scholar]
- Gutzeit HO, Arendt D. Blocked endocytotic uptake by the oocyte causes accumulation of vitellogenins in the haemolymph of the female-sterile mutants quitPX61 and stand stillPS34 of Drosophila. Cell Tissue Res. 1994;275:291–298. doi: 10.1007/BF00319427. [DOI] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55:577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Dass S, Kaltschmidt JA, Lopez-Schier H, St Johnston D, Brand AH, Roth S, Guichet A. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr. Biol. 2002;12:1971–1981. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Koch EA, Spitzer RH. Multiple effects of colchicine on oogenesis in Drosophila: induced sterility and switch of potential oocyte to nurse-cell developmental pathway. Cell Tissue Res. 1983;228:21–32. doi: 10.1007/BF00206261. [DOI] [PubMed] [Google Scholar]
- Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- Liang L, Diehl-Jones W, Lasko P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc. Natl. Acad. Sci. USA. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126:5353–5364. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- Mallik R, Gross SP. Molecular motors: strategies to get along. Curr. Biol. 2004;14:R971–R982. doi: 10.1016/j.cub.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Manseau L, Calley J, Phan H. Profilin is required for posterior patterning of the Drosophila oocyte. Development. 1996;122:2109–2116. doi: 10.1242/dev.122.7.2109. [DOI] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, Leclerc V, Smith-Litiere K, St Johnston D. The identification of novel genes required for Drosophila anteroposterior axis formation in a germline clone screen using GFP-Staufen. Development. 2003;130:4201–4215. doi: 10.1242/dev.00630. [DOI] [PubMed] [Google Scholar]
- McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Toba S, Edamatsu M, Watai-Nishii J, Hirokawa N, Toyoshima YY, Kikkawa M. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 2004;23:2459–2467. doi: 10.1038/sj.emboj.7600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Otto IM, Raabe T, Rennefahrt UE, Bork P, Rapp UR, Kerkhoff E. The p150-Spir protein provides a link between c-Jun N-terminal kinase function and actin reorganization. Curr. Biol. 2000;10:345–348. doi: 10.1016/s0960-9822(00)00388-2. [DOI] [PubMed] [Google Scholar]
- Palacios IM, St Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development. 2002;129:5473–5485. doi: 10.1242/dev.00119. [DOI] [PubMed] [Google Scholar]
- Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat. Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Saxton WM. Microtubules, motors, and mRNA localization mechanisms: watching fluorescent messages move. Cell. 2001;107:707–710. doi: 10.1016/s0092-8674(01)00602-x. [DOI] [PubMed] [Google Scholar]
- Saxton WM, Hicks J, Goldstein LSB, Raff EC. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991;64:1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- Shimmen T, Yokota E. Cytoplasmic streaming in plants. Curr. Opin. Cell Biol. 2004;16:68–72. doi: 10.1016/j.ceb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE. Immunofluorescence analysis of the cytoskeleton during oogenesis and early embryogenesis. Methods Cell Biol. 1994a;44:489–505. doi: 10.1016/s0091-679x(08)60928-0. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE. Premature microtubule-dependent cytoplasmic streaming in cappuccino and spire mutant oocytes. Science. 1994b;265:2093–2096. doi: 10.1126/science.8091233. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Hazelrigg TI. In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway. Development. 1998;125:3655–3666. doi: 10.1242/dev.125.18.3655. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992;115:923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Verheyen EM, Cooley L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development. 1994;120:717–728. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
- Warn RM, Gutzeit HO, Smith L, Warn A. F-actin rings are associated with the Drosohpila egg chamber canals. Exp. Cell Res. 1985;157:355–363. doi: 10.1016/0014-4827(85)90120-x. [DOI] [PubMed] [Google Scholar]
- Wellington A, Emmons S, James B, Calley J, Grover M, Tolias P, Manseau L. Spire contains actin binding domains and is related to ascidian posterior end mark-5. Development. 1999;126:5267–5274. doi: 10.1242/dev.126.23.5267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


