Abstract
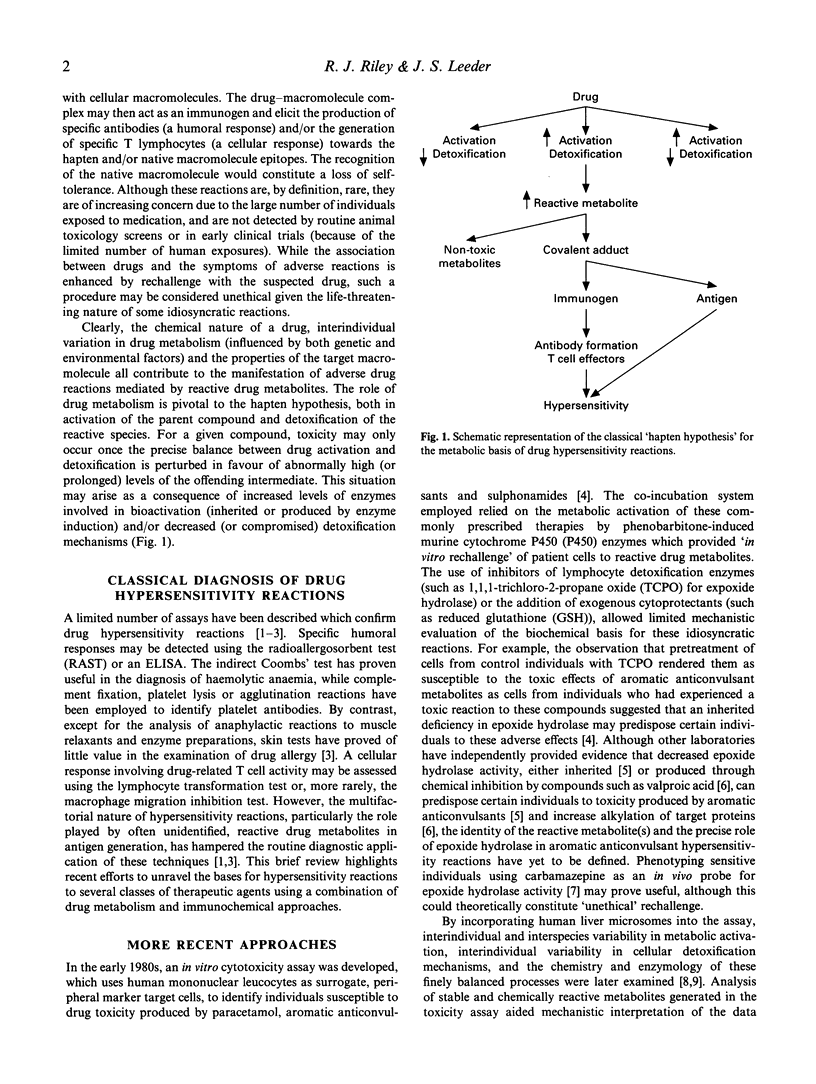
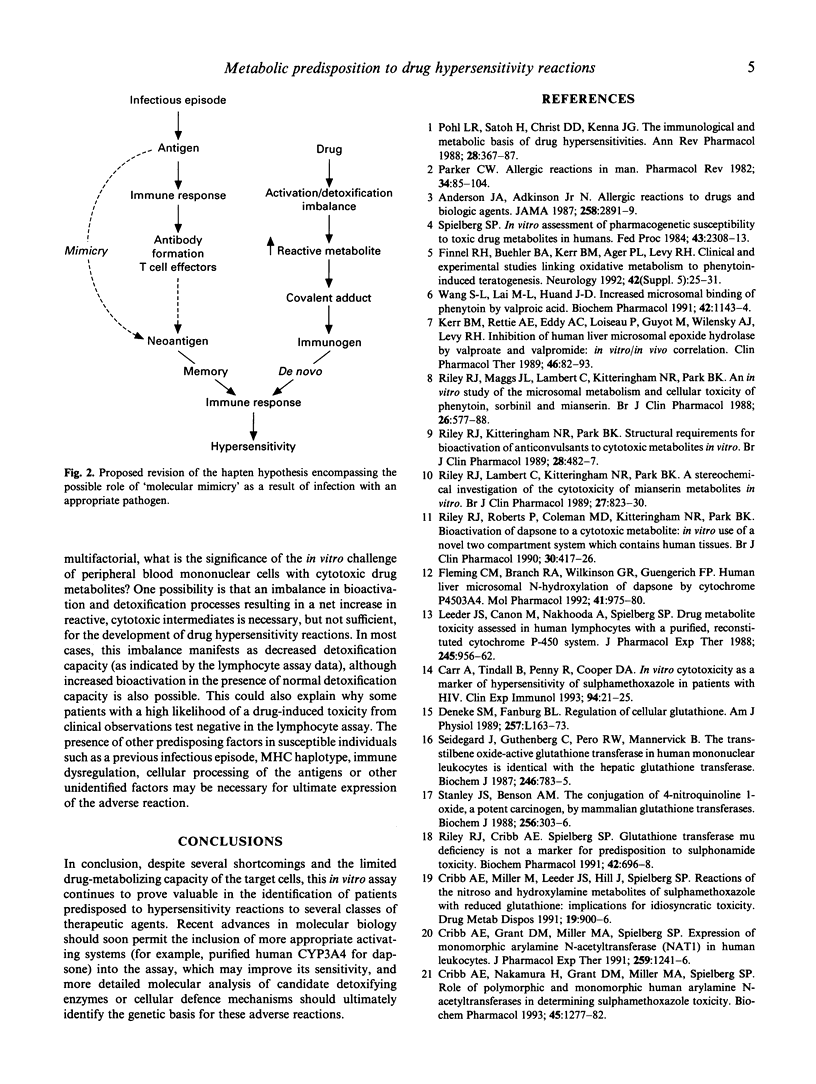
Idiosyncratic hypersensitivity reactions may account for up to 25% of all adverse reactions, and pose a constant problem to physicians because of their unpredictable nature, potentially fatal outcome and resemblance to other disease processes. Current understanding of how drug allergy arises is based largely on the hapten hypothesis: since most drugs are not chemically reactive per se, they must be activated metabolically to reactive species which may become immunogenic through interactions with cellular macromolecules. The role of drug metabolism is thus pivotal to the hapten hypothesis both in activation of the parent compound and detoxification of the reactive species. Although conjugation reactions may occasionally produce potential immunogens (for example, the generation of acylglucuronides from non-steroidal anti-inflammatory drugs such as diclofenac), bioactivation is catalysed most frequently by cytochrome P450 (P450) enzymes. The multifactorial nature of hypersensitivity reactions, particularly the role of often unidentified, reactive drug metabolites in antigen generation, has hampered the routine diagnosis of these disorders by classical immunological methods designed to detect circulating antibodies or sensitized T cells. Similarly, species differences in drug metabolism and immune system regulation have largely precluded the establishment of appropriate animal models with which to examine the immunopathological mechanisms of these toxicities. However, the combined use of in vitro toxicity assays incorporating human tissues and in vivo phenotyping (or, ultimately, in vitro genotyping) methods for drug detoxification pathways may provide the metabolic basis for hypersensitivity reactions to several drugs. This brief review highlights recent efforts to unravel the bases for hypersensitivity reactions to these therapeutic agents (which include anticonvulsants and sulphonamides) using drug metabolism and immunochemical approaches. In particular, examples are provided which illustrate breakthroughs in the identification of the chemical nature of the reactive metabolites which become bound to cellular macromolecules, the enzyme systems responsible for their generation and (possibly) detoxification, and the target proteins implicated in the subsequent immune response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarli J. A., Fontana A. Immunological aspects of epilepsy. Epilepsia. 1980 Oct;21(5):451–457. doi: 10.1111/j.1528-1157.1980.tb04295.x. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Adkinson N. F., Jr Allergic reactions to drugs and biologic agents. JAMA. 1987 Nov 27;258(20):2891–2899. [PubMed] [Google Scholar]
- Baum H., Butler P., Davies H., Sternberg M. J., Burroughs A. K. Autoimmune disease and molecular mimicry: an hypothesis. Trends Biochem Sci. 1993 Apr;18(4):140–144. doi: 10.1016/0968-0004(93)90022-f. [DOI] [PubMed] [Google Scholar]
- Beaune P., Dansette P. M., Mansuy D., Kiffel L., Finck M., Amar C., Leroux J. P., Homberg J. C. Human anti-endoplasmic reticulum autoantibodies appearing in a drug-induced hepatitis are directed against a human liver cytochrome P-450 that hydroxylates the drug. Proc Natl Acad Sci U S A. 1987 Jan;84(2):551–555. doi: 10.1073/pnas.84.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelsterli U. A. Specific targets of covalent drug-protein interactions in hepatocytes and their toxicological significance in drug-induced liver injury. Drug Metab Rev. 1993;25(4):395–451. doi: 10.3109/03602539308993981. [DOI] [PubMed] [Google Scholar]
- Bourdi M., Gautier J. C., Mircheva J., Larrey D., Guillouzo A., Andre C., Belloc C., Beaune P. H. Anti-liver microsomes autoantibodies and dihydralazine-induced hepatitis: specificity of autoantibodies and inductive capacity of the drug. Mol Pharmacol. 1992 Aug;42(2):280–285. [PubMed] [Google Scholar]
- Burroughs A. K., Butler P., Sternberg M. J., Baum H. Molecular mimicry in liver disease. Nature. 1992 Jul 30;358(6385):377–378. doi: 10.1038/358377a0. [DOI] [PubMed] [Google Scholar]
- Carr A., Tindall B., Penny R., Cooper D. A. In vitro cytotoxicity as a marker of hypersensitivity to sulphamethoxazole in patients with HIV. Clin Exp Immunol. 1993 Oct;94(1):21–25. doi: 10.1111/j.1365-2249.1993.tb05971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribb A. E., Grant D. M., Miller M. A., Spielberg S. P. Expression of monomorphic arylamine N-acetyltransferase (NAT1) in human leukocytes. J Pharmacol Exp Ther. 1991 Dec;259(3):1241–1246. [PubMed] [Google Scholar]
- Cribb A. E., Miller M., Leeder J. S., Hill J., Spielberg S. P. Reactions of the nitroso and hydroxylamine metabolites of sulfamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab Dispos. 1991 Sep-Oct;19(5):900–906. [PubMed] [Google Scholar]
- Cribb A. E., Nakamura H., Grant D. M., Miller M. A., Spielberg S. P. Role of polymorphic and monomorphic human arylamine N-acetyltransferases in determining sulfamethoxazole metabolism. Biochem Pharmacol. 1993 Mar 24;45(6):1277–1282. doi: 10.1016/0006-2952(93)90280-a. [DOI] [PubMed] [Google Scholar]
- Finnell R. H., Buehler B. A., Kerr B. M., Ager P. L., Levy R. H. Clinical and experimental studies linking oxidative metabolism to phenytoin-induced teratogenesis. Neurology. 1992 Apr;42(4 Suppl 5):25–31. [PubMed] [Google Scholar]
- Fleming C. M., Branch R. A., Wilkinson G. R., Guengerich F. P. Human liver microsomal N-hydroxylation of dapsone by cytochrome P-4503A4. Mol Pharmacol. 1992 May;41(5):975–980. [PubMed] [Google Scholar]
- Gueguen M., Boniface O., Bernard O., Clerc F., Cartwright T., Alvarez F. Identification of the main epitope on human cytochrome P450 IID6 recognized by anti-liver kidney microsome antibody. J Autoimmun. 1991 Aug;4(4):607–615. doi: 10.1016/0896-8411(91)90180-k. [DOI] [PubMed] [Google Scholar]
- Gut J., Christen U., Huwyler J. Mechanisms of halothane toxicity: novel insights. Pharmacol Ther. 1993;58(2):133–155. doi: 10.1016/0163-7258(93)90047-h. [DOI] [PubMed] [Google Scholar]
- Kenna J. G., Knight T. L., van Pelt F. N. Immunity to halothane metabolite-modified proteins in halothane hepatitis. Ann N Y Acad Sci. 1993 Jun 23;685:646–661. doi: 10.1111/j.1749-6632.1993.tb35930.x. [DOI] [PubMed] [Google Scholar]
- Kerr B. M., Rettie A. E., Eddy A. C., Loiseau P., Guyot M., Wilensky A. J., Levy R. H. Inhibition of human liver microsomal epoxide hydrolase by valproate and valpromide: in vitro/in vivo correlation. Clin Pharmacol Ther. 1989 Jul;46(1):82–93. doi: 10.1038/clpt.1989.110. [DOI] [PubMed] [Google Scholar]
- Krohn K., Uibo R., Aavik E., Peterson P., Savilahti K. Identification by molecular cloning of an autoantigen associated with Addison's disease as steroid 17 alpha-hydroxylase. Lancet. 1992 Mar 28;339(8796):770–773. doi: 10.1016/0140-6736(92)91894-e. [DOI] [PubMed] [Google Scholar]
- Leeder J. S., Cannon M., Nakhooda A., Spielberg S. P. Drug metabolite toxicity assessed in human lymphocytes with a purified, reconstituted cytochrome P-450 system. J Pharmacol Exp Ther. 1988 Jun;245(3):956–962. [PubMed] [Google Scholar]
- Leeder J. S., Riley R. J., Cook V. A., Spielberg S. P. Human anti-cytochrome P450 antibodies in aromatic anticonvulsant-induced hypersensitivity reactions. J Pharmacol Exp Ther. 1992 Oct;263(1):360–367. [PubMed] [Google Scholar]
- Leung P. S., Gershwin M. E. The molecular structure of autoantigens. Curr Opin Immunol. 1989;2(4):567–575. doi: 10.1016/0952-7915(90)90012-6. [DOI] [PubMed] [Google Scholar]
- Loeper J., Descatoire V., Maurice M., Beaune P., Belghiti J., Houssin D., Ballet F., Feldmann G., Guengerich F. P., Pessayre D. Cytochromes P-450 in human hepatocyte plasma membrane: recognition by several autoantibodies. Gastroenterology. 1993 Jan;104(1):203–216. doi: 10.1016/0016-5085(93)90853-5. [DOI] [PubMed] [Google Scholar]
- Manns M. P., Griffin K. J., Quattrochi L. C., Sacher M., Thaler H., Tukey R. H., Johnson E. F. Identification of cytochrome P450IA2 as a human autoantigen. Arch Biochem Biophys. 1990 Jul;280(1):229–232. doi: 10.1016/0003-9861(90)90541-6. [DOI] [PubMed] [Google Scholar]
- Manns M. P., Griffin K. J., Sullivan K. F., Johnson E. F. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. J Clin Invest. 1991 Oct;88(4):1370–1378. doi: 10.1172/JCI115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzechowska-Juzwenko K., Milejski P., Patkowski J., Nittner-Marszalska M., Malolepszy J. Acetylator phenotype in patients with allergic diseases and its clinical significance. Int J Clin Pharmacol Ther Toxicol. 1990 Oct;28(10):420–425. [PubMed] [Google Scholar]
- Parker C. W. Allergic reactions in man. Pharmacol Rev. 1982 Mar;34(1):85–104. [PubMed] [Google Scholar]
- Pirmohamed M., Kitteringham N. R., Breckenridge A. M., Park B. K. The effect of enzyme induction on the cytochrome P450-mediated bioactivation of carbamazepine by mouse liver microsomes. Biochem Pharmacol. 1992 Dec 15;44(12):2307–2314. doi: 10.1016/0006-2952(92)90674-8. [DOI] [PubMed] [Google Scholar]
- Pohl L. R., Satoh H., Christ D. D., Kenna J. G. The immunologic and metabolic basis of drug hypersensitivities. Annu Rev Pharmacol Toxicol. 1988;28:367–387. doi: 10.1146/annurev.pa.28.040188.002055. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Cribb A. E., Spielberg S. P. Glutathione transferase mu deficiency is not a marker for predisposition to sulphonamide toxicity. Biochem Pharmacol. 1991 Jul 15;42(3):696–698. doi: 10.1016/0006-2952(91)90334-2. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Kitteringham N. R., Park B. K. Structural requirements for bioactivation of anticonvulsants to cytotoxic metabolites in vitro. Br J Clin Pharmacol. 1989 Oct;28(4):482–487. doi: 10.1111/j.1365-2125.1989.tb03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Lambert C., Kitteringham N. R., Park B. K. A stereochemical investigation of the cytotoxicity of mianserin metabolites in vitro. Br J Clin Pharmacol. 1989 Jun;27(6):823–830. doi: 10.1111/j.1365-2125.1989.tb03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Maggs J. L., Lambert C., Kitteringham N. R., Park B. K. An in vitro study of the microsomal metabolism and cellular toxicity of phenytoin, sorbinil and mianserin. Br J Clin Pharmacol. 1988 Nov;26(5):577–588. doi: 10.1111/j.1365-2125.1988.tb05298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Roberts P., Coleman M. D., Kitteringham N. R., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite: in vitro use of a novel two compartment system which contains human tissues. Br J Clin Pharmacol. 1990 Sep;30(3):417–426. doi: 10.1111/j.1365-2125.1990.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Smith G., Wolf C. R., Cook V. A., Leeder J. S. Human anti-endoplasmic reticulum autoantibodies produced in aromatic anticonvulsant hypersensitivity reactions recognise rodent CYP3A proteins and a similarly regulated human P450 enzyme(s) Biochem Biophys Res Commun. 1993 Feb 26;191(1):32–40. doi: 10.1006/bbrc.1993.1180. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Guthenberg C., Pero R. W., Mannervik B. The trans-stilbene oxide-active glutathione transferase in human mononuclear leucocytes is identical with the hepatic glutathione transferase mu. Biochem J. 1987 Sep 15;246(3):783–785. doi: 10.1042/bj2460783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg S. P. In vitro assessment of pharmacogenetic susceptibility to toxic drug metabolites in humans. Fed Proc. 1984 May 15;43(8):2308–2313. [PubMed] [Google Scholar]
- Stanley J. S., Benson A. M. The conjugation of 4-nitroquinoline 1-oxide, a potent carcinogen, by mammalian glutathione transferases. 4-Nitroquinoline 1-oxide conjugation by human, rat and mouse liver cytosols, extrahepatic organs of mice and purified mouse glutathione transferase isoenzymes. Biochem J. 1988 Nov 15;256(1):303–306. doi: 10.1042/bj2560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban G., Speerschneider P., Dekant W. Metabolism of the chlorofluorocarbon substitute 1,1-dichloro-2,2,2-trifluoroethane by rat and human liver microsomes: the role of cytochrome P450 2E1. Chem Res Toxicol. 1994 Mar-Apr;7(2):170–176. doi: 10.1021/tx00038a009. [DOI] [PubMed] [Google Scholar]
- Vergani D., Mieli-Vergani G. Type II autoimmune hepatitis. What is the role of the hepatitis C virus? Gastroenterology. 1993 Jun;104(6):1870–1873. doi: 10.1016/0016-5085(93)90673-z. [DOI] [PubMed] [Google Scholar]
- Vergani D., Mieli-Vergani G. Type II autoimmune hepatitis: the conundrum of cytochrome P450IID6. Clin Exp Immunol. 1993 Jun;92(3):367–368. doi: 10.1111/j.1365-2249.1993.tb03406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M. E., Doecke C. J., Mackenzie P. I., McManus M. E., Miners J. O., Rees D. L., Gasser R., Meyer U. A., Birkett D. J. Site-directed mutation studies of human liver cytochrome P-450 isoenzymes in the CYP2C subfamily. Biochem J. 1993 Jan 15;289(Pt 2):533–538. doi: 10.1042/bj2890533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. L., Lai M. L., Huang J. D. Increased microsomal irreversible binding of phenytoin by valproic acid. Biochem Pharmacol. 1991 Aug 8;42(5):1143–1144. [PubMed] [Google Scholar]
- Winqvist O., Gustafsson J., Rorsman F., Karlsson F. A., Kämpe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison's disease. J Clin Invest. 1993 Nov;92(5):2377–2385. doi: 10.1172/JCI116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A. M., Mura C., De Lemos-Chiarandini C., Krishnamoorthy R., Alvarez F. Cytochrome P450IID6 recognized by LKM1 antibody is not exposed on the surface of hepatocytes. Clin Exp Immunol. 1993 Jun;92(3):381–390. doi: 10.1111/j.1365-2249.1993.tb03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger U. M., Hauri H. P., Loeper J., Homberg J. C., Meyer U. A. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]


