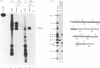Abstract
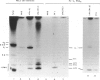
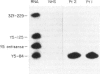
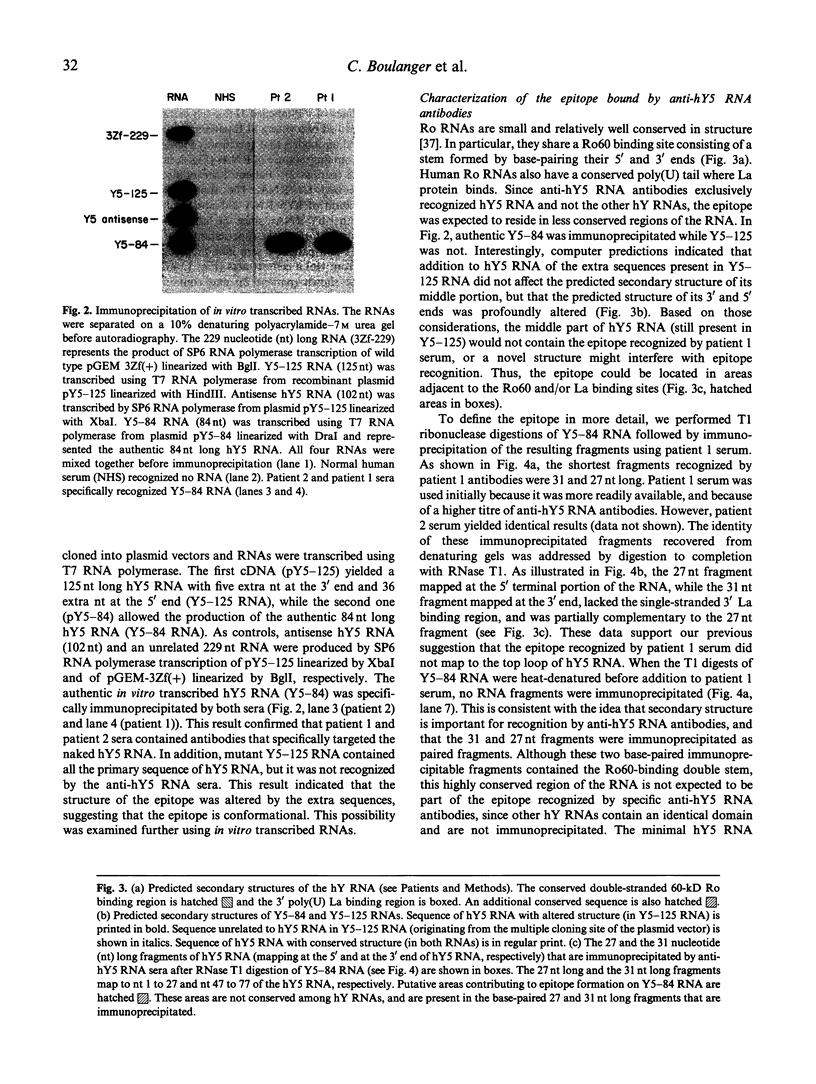
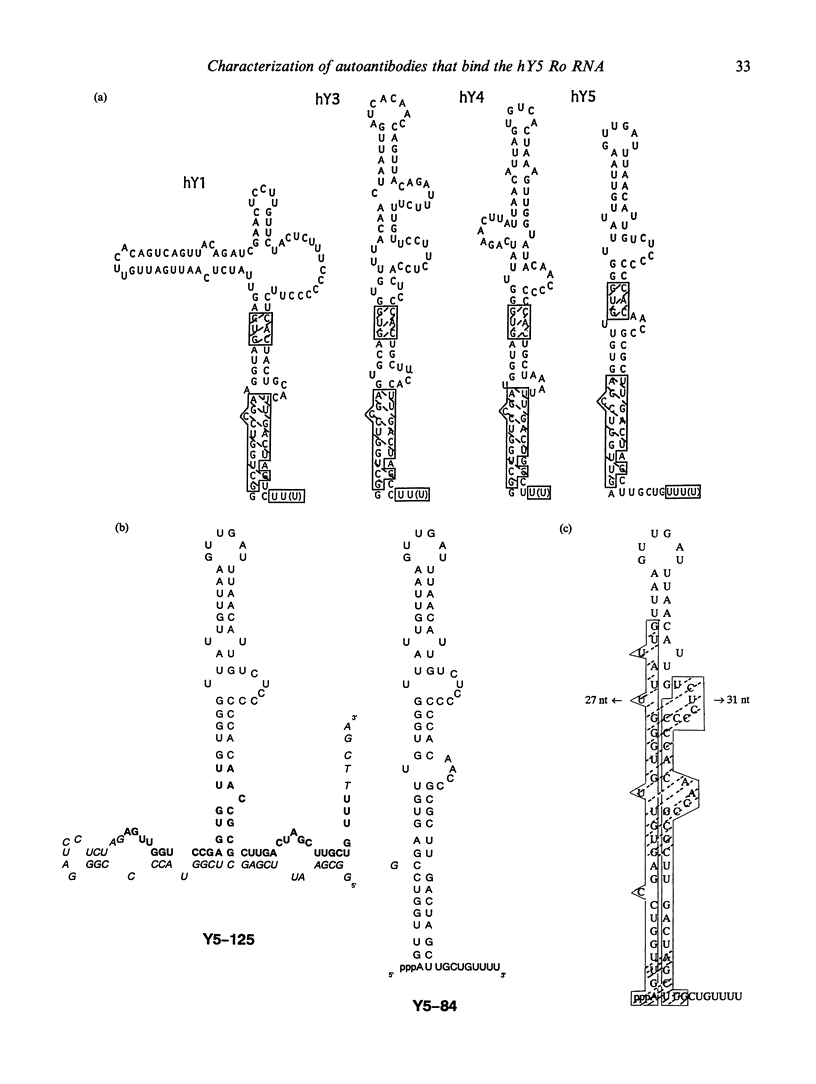
We report the existence of a novel autoantibody specificity linked to anti-Ro antibodies. Sera from two patients with anti-Ro ribonucleoprotein (RNP) antibodies also contained antibodies that immunoprecipitated specifically either the deproteinized RNA component of the RohY5 RNP particle, or intact in vitro transcribed hY5 RNA. No serum recognized specifically the other hY RNAs. A mutant hY5 RNA with additional nucleotides (nt) at both extremities was not immunoprecipitated, possibly because of altered secondary structure. Following digestion of hY5 RNA with ribonuclease T1, the smallest immunoprecipitable RNA fragments were 27 and 31 nt long, and respectively mapped to the 5' and 3' ends of hY5 RNA, excluding the La-binding region. Base pairing between the 27 and 31 nt long fragments was required for recognition by antibodies. Our data indicate that the epitope bound by anti-hY5 RNA antibodies is conformational. We have previously reported that most anti-Ro sera contain a population of antibodies specific for the RohY5 RNP. Since antibodies to the deproteinized hY RNAs within anti-Ro sera are also restricted to anti-hY5 RNA, a direct role for the human-specific RohY5 particles in the immunization process leading to the production of anti-Ro antibodies is suggested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D., Fishbein E., García-Ortigoza E., Estrada-Parra S. Uracil-specific anti-R.N.A. antibodies in scleroderma. Lancet. 1975 Feb 15;1(7903):363–366. doi: 10.1016/s0140-6736(75)91279-9. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Craft J. Biochemical and immunological heterogeneity of the Ro ribonucleoprotein particles. Analysis with sera specific for the RohY5 particle. J Clin Invest. 1989 Jul;84(1):270–279. doi: 10.1172/JCI114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Craft J. Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Invest. 1990 Apr;85(4):1182–1190. doi: 10.1172/JCI114551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Lopez-Longo F. J., Lapointe S., Ménard H. A. Sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum. 1991 Jun;34(6):722–730. doi: 10.1002/art.1780340613. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. C., Mathews M. B. Autoreactive epitope defined as the anticodon region of alanine transfer RNA. Science. 1987 Nov 20;238(4830):1116–1119. doi: 10.1126/science.2446387. [DOI] [PubMed] [Google Scholar]
- Buyon J. P., Slade S. G., Chan E. K., Tan E. M., Winchester R. Effective separation of the 52 kDa SSA/Ro polypeptide from the 48 kDa SSB/La polypeptide by altering conditions of polyacrylamide gel electrophoresis. J Immunol Methods. 1990 May 25;129(2):207–210. doi: 10.1016/0022-1759(90)90440-7. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Hamel J. C., Buyon J. P., Tan E. M. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest. 1991 Jan;87(1):68–76. doi: 10.1172/JCI115003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. L., Brot N., Weissbach H., Elkon K. Lupus antiribosomal P antisera contain antibodies to a small fragment of 28S rRNA located in the proposed ribosomal GTPase center. J Exp Med. 1991 Sep 1;174(3):507–514. doi: 10.1084/jem.174.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Keene J. D. A sequence-specific conformational epitope on U1 RNA is recognized by a unique autoantibody. Proc Natl Acad Sci U S A. 1988 May;85(10):3299–3303. doi: 10.1073/pnas.85.10.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Steinberg A. D., Schechter A. N. The reaction of SLE antibodies with native, single stranded RNA: radioassay and binding specificities. J Immunol. 1978 Feb;120(2):550–557. [PubMed] [Google Scholar]
- Frank M. B., Itoh K., McCubbin V. Epitope mapping of the 52-kD Ro/SSA autoantigen. Clin Exp Immunol. 1994 Mar;95(3):390–396. doi: 10.1111/j.1365-2249.1994.tb07009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Hoet R. M., Koornneef I., de Rooij D. J., van de Putte L. B., van Venrooij W. J. Changes in anti-U1 RNA antibody levels correlate with disease activity in patients with systemic lupus erythematosus overlap syndrome. Arthritis Rheum. 1992 Oct;35(10):1202–1210. doi: 10.1002/art.1780351013. [DOI] [PubMed] [Google Scholar]
- Hoet R. M., van Venrooij W. J. B-cell epitopes of RNA autoantigens. Mol Biol Rep. 1992 Jun;16(3):199–205. doi: 10.1007/BF00464708. [DOI] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Kelekar A., Saitta M. R., Keene J. D. Molecular composition of Ro small ribonucleoprotein complexes in human cells. Intracellular localization of the 60- and 52-kD proteins. J Clin Invest. 1994 Apr;93(4):1637–1644. doi: 10.1172/JCI117145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan C., Adams S., Stanik V., Datta S. K. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993 May 1;177(5):1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C. A., Harley J. B. A subset of hY RNAs is associated with erythrocyte Ro ribonucleoproteins. EMBO J. 1990 Nov;9(11):3683–3689. doi: 10.1002/j.1460-2075.1990.tb07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C. A., Margelot K., Wolin S. L. Xenopus Ro ribonucleoproteins: members of an evolutionarily conserved class of cytoplasmic ribonucleoproteins. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7250–7254. doi: 10.1073/pnas.90.15.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano Y., Medsger T. A., Jr Novel human autoantibodies reactive with 5'-terminal trimethylguanosine cap structures of U small nuclear RNA. J Immunol. 1992 Aug 1;149(3):1093–1098. [PubMed] [Google Scholar]
- Plotz P. H. The role of autoantigens in the induction and maintenance of autoimmunity. Mol Biol Rep. 1992 Jun;16(3):127–132. doi: 10.1007/BF00464699. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Significance of the Ro antigen system. J Clin Immunol. 1986 Sep;6(5):339–348. doi: 10.1007/BF00915372. [DOI] [PubMed] [Google Scholar]
- Slobbe R. L., Pluk W., van Venrooij W. J., Pruijn G. J. Ro ribonucleoprotein assembly in vitro. Identification of RNA-protein and protein-protein interactions. J Mol Biol. 1992 Sep 20;227(2):361–366. doi: 10.1016/0022-2836(92)90890-v. [DOI] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. The experimental induction of antibodies to nucleic acids. Methods Enzymol. 1980;70(A):70–85. doi: 10.1016/s0076-6879(80)70042-3. [DOI] [PubMed] [Google Scholar]
- Sturgess A. D., Peterson M. G., McNeilage L. J., Whittingham S., Coppel R. L. Characteristics and epitope mapping of a cloned human autoantigen La. J Immunol. 1988 May 1;140(9):3212–3218. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Keene J. D. Autoantibodies specific for U1 RNA and initiator methionine tRNA. J Biol Chem. 1986 Apr 25;261(12):5467–5472. [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Hoet R., Castrop J., Hageman B., Mattaj I. W., van de Putte L. B. Anti-(U1) small nuclear RNA antibodies in anti-small nuclear ribonucleoprotein sera from patients with connective tissue diseases. J Clin Invest. 1990 Dec;86(6):2154–2160. doi: 10.1172/JCI114954. [DOI] [PMC free article] [PubMed] [Google Scholar]