Abstract
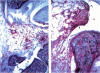
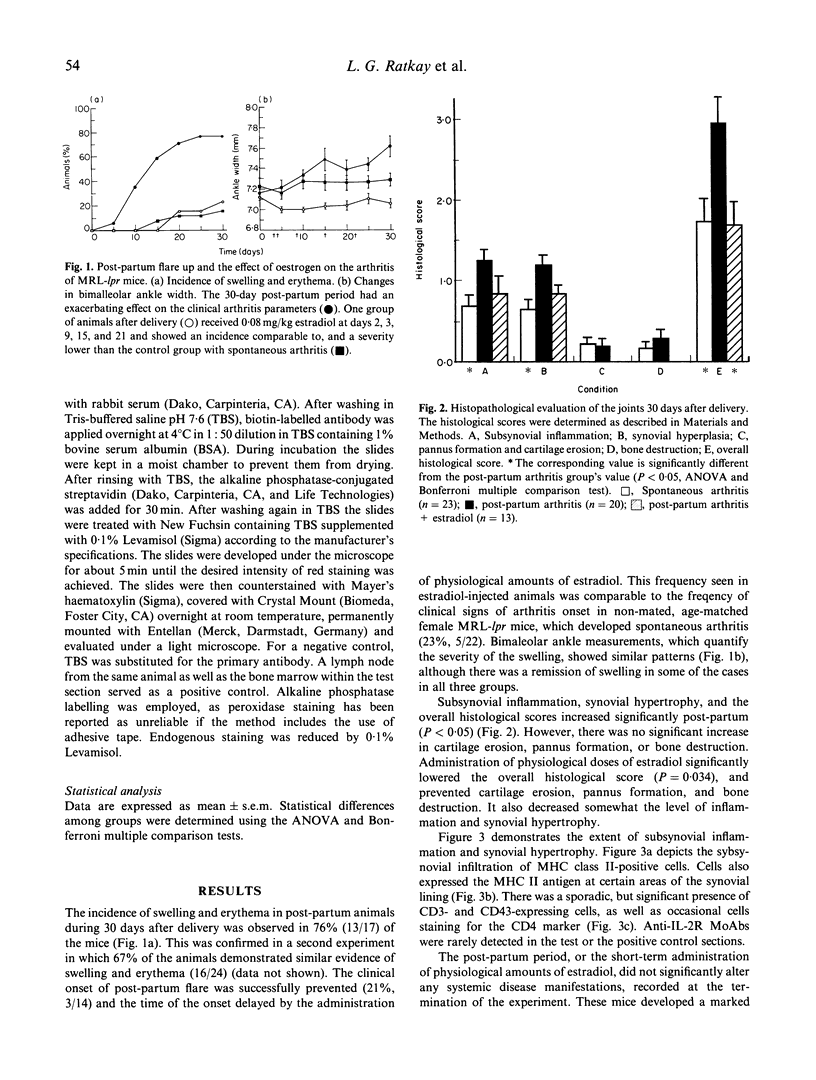
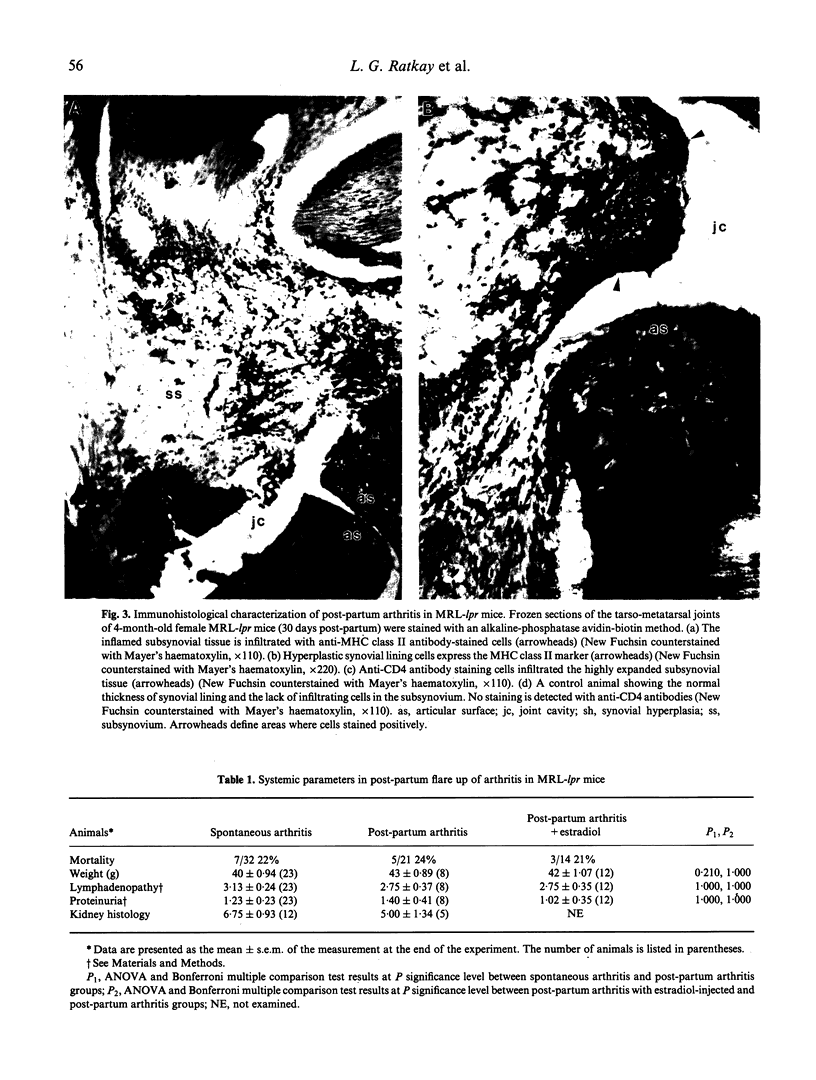
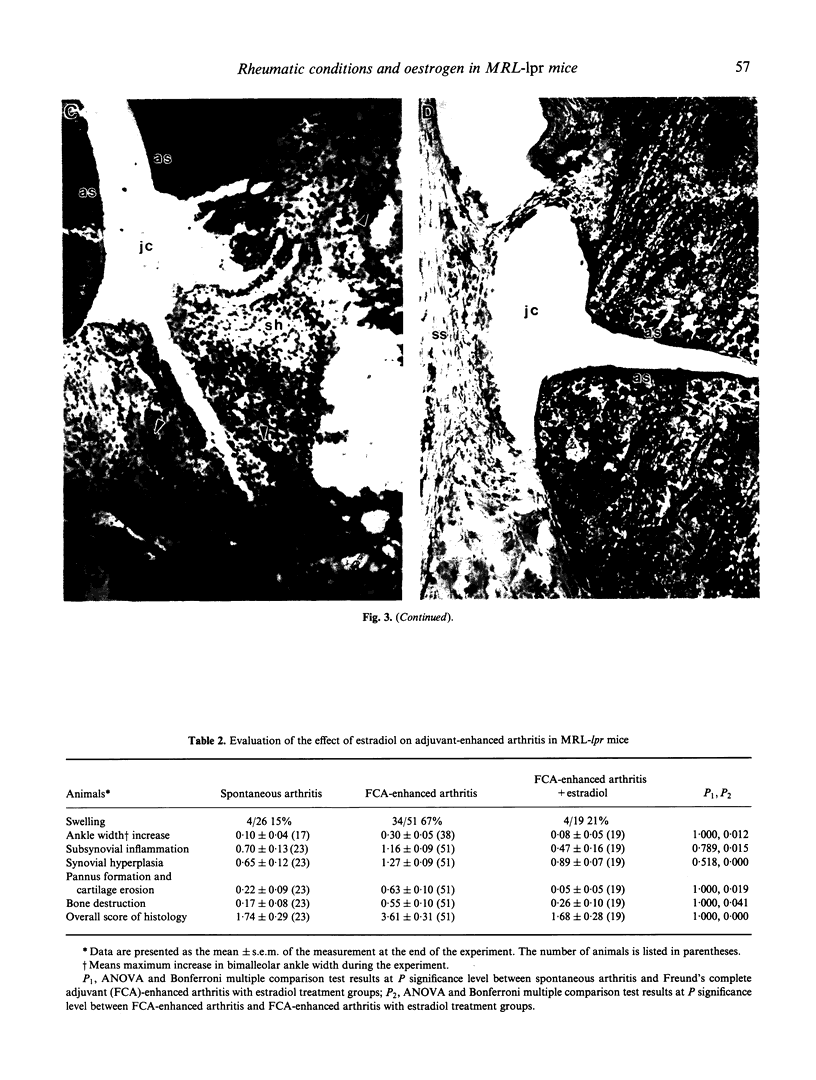
Sixty-eight percent of female MRL-lpr mice developed a post-partum exacerbation of their mild spontaneous arthritis within 30 days of parturition. The flare became evident between 5 and 15 days after delivery. Histologically it was characterized by a significant increase of subsynovial inflammation and synovial hyperplasia without changes in the level of cartilage and bone erosion. Immunohistologically, marked subsynovial and frequent synovial staining of MHC class II bearing cells was noted, along with the sporadic presence of CD3, CD4, and CD43 receptor-bearing cells in the subsynovium. Injection of physiological levels (0.08 mg/kg) of estradiol on days 2, 3, 9, 15 and 20 post-partum delayed and reduced the flare to 23% of the animals. Administration of pharmacological amounts (0.4 mg/kg per day for 2 weeks following Freund's complete adjuvant injection) prevented adjuvant-enhanced arthritis, reducing the incidence from 67% to the baseline 21% level. Deleterious changes in the underlying systemic lupus erythematosus (SLE), as demonstrated by proteinuria and mortality rate increases, were elicited only by the employed pharmacological amounts of estradiol. These results indicate that the MRL-lpr mice might serve as a model for post-partum flare of arthritis in SLE and rheumatoid arthritis by providing an approach to study the complexity of the effects of pregnancy on autoimmune diseases, and to obtain further evidence for the involvement of oestrogen in arthritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar Ahmed S., Dauphinee M. J., Talal N. Effects of short-term administration of sex hormones on normal and autoimmune mice. J Immunol. 1985 Jan;134(1):204–210. [PubMed] [Google Scholar]
- Berczi I., Nagy E. A possible role of prolactin in adjuvant arthritis. Arthritis Rheum. 1982 May;25(5):591–594. doi: 10.1002/art.1780250517. [DOI] [PubMed] [Google Scholar]
- Bijlsma J. W., Van den Brink H. R. Estrogens and rheumatoid arthritis. Am J Reprod Immunol. 1992 Oct-Dec;28(3-4):231–234. doi: 10.1111/j.1600-0897.1992.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Brick J. E., Walker S. E., Wise K. S. Hormone control of autoantibodies to calf thymus nuclear extract (CTE) and DNA in MRL-lpr and MRL-+/+ mice. Clin Immunol Immunopathol. 1988 Jan;46(1):68–81. doi: 10.1016/0090-1229(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Buzás E. I., Holló K., Rubliczky L., Garzó M., Nyirkos P., Glant T. T. Effect of pregnancy on proteoglycan-induced progressive polyarthritis in BALB/c mice: remission of disease activity. Clin Exp Immunol. 1993 Nov;94(2):252–260. doi: 10.1111/j.1365-2249.1993.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten H., Holmdahl R., Tarkowski A., Nilsson L. A. Oestradiol- and testosterone-mediated effects on the immune system in normal and autoimmune mice are genetically linked and inherited as dominant traits. Immunology. 1989 Oct;68(2):209–214. [PMC free article] [PubMed] [Google Scholar]
- Carlsten H., Nilsson N., Jonsson R., Bäckman K., Holmdahl R., Tarkowski A. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell Immunol. 1992 Oct 1;144(1):190–202. doi: 10.1016/0008-8749(92)90236-i. [DOI] [PubMed] [Google Scholar]
- Carlsten H., Nilsson N., Jonsson R., Tarkowski A. Differential effects of oestrogen in murine lupus: acceleration of glomerulonephritis and amelioration of T cell-mediated lesions. J Autoimmun. 1991 Dec;4(6):845–856. doi: 10.1016/0896-8411(91)90048-h. [DOI] [PubMed] [Google Scholar]
- Carlsten H., Tarkowski A., Holmdahl R., Nilsson L. A. Oestrogen is a potent disease accelerator in SLE-prone MRL lpr/lpr mice. Clin Exp Immunol. 1990 Jun;80(3):467–473. doi: 10.1111/j.1365-2249.1990.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon F. J., Andrews B. S., Eisenberg R. A., McConahey P. J., Theofilopoulos A. N., Wilson C. B. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978 Jun;21(5 Suppl):S64–S67. doi: 10.1002/art.1780210909. [DOI] [PubMed] [Google Scholar]
- Hang L. M., Aguado M. T., Dixon F. J., Theofilopoulos A. N. Induction of severe autoimmune disease in normal mice by simultaneous action of multiple immunostimulators. J Exp Med. 1985 Feb 1;161(2):423–428. doi: 10.1084/jem.161.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Dixon F. J. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982 Jun 1;155(6):1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazes J. M. Pregnancy and its effect on the risk of developing rheumatoid arthritis. Ann Rheum Dis. 1991 Feb;50(2):71–72. doi: 10.1136/ard.50.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazes J. M., van Zeben D. Oral contraception and its possible protection against rheumatoid arthritis. Ann Rheum Dis. 1991 Feb;50(2):72–74. doi: 10.1136/ard.50.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Carlsten H., Jansson L., Larsson P. Oestrogen is a potent immunomodulator of murine experimental rheumatoid disease. Br J Rheumatol. 1989;28 (Suppl 1):54–71. doi: 10.1093/rheumatology/xxviii.suppl_1.54. [DOI] [PubMed] [Google Scholar]
- Holmdahl R. Estrogen exaggerates lupus but suppresses T-cell-dependent autoimmune disease. J Autoimmun. 1989 Oct;2(5):651–656. doi: 10.1016/s0896-8411(89)80004-6. [DOI] [PubMed] [Google Scholar]
- Jansson L., Holmdahl R. Oestrogen induced suppression of collagen arthritis. IV: Progesterone alone does not affect the course of arthritis but enhances the oestrogen-mediated therapeutic effect. J Reprod Immunol. 1989 May;15(2):141–150. doi: 10.1016/0165-0378(89)90033-8. [DOI] [PubMed] [Google Scholar]
- Jansson L., Mattsson A., Mattsson R., Holmdahl R. Estrogen induced suppression of collagen arthritis. V: Physiological level of estrogen in DBA/1 mice is therapeutic on established arthritis, suppresses anti-type II collagen T-cell dependent immunity and stimulates polyclonal B-cell activity. J Autoimmun. 1990 Jun;3(3):257–270. doi: 10.1016/0896-8411(90)90145-i. [DOI] [PubMed] [Google Scholar]
- Mattsson R., Mattsson A., Holmdahl R., Whyte A., Rook G. A. Maintained pregnancy levels of oestrogen afford complete protection from post-partum exacerbation of collagen-induced arthritis. Clin Exp Immunol. 1991 Jul;85(1):41–47. doi: 10.1111/j.1365-2249.1991.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie W. N., Jr, Sunderrajan E. V., Kavanaugh J. L., Braun S., Ansbacher L., Walker S. E. Sex hormones modulate the response of pulmonary perivascular inflammation to cyclophosphamide therapy in MRL/MpJ-lpr/lpr mice. Am J Pathol. 1988 Jun;131(3):530–538. [PMC free article] [PubMed] [Google Scholar]
- Meacock S. C., Brandon D. R., Brown C. P., Swann B. P. A novel technique for immunohistoperoxidase staining of unfixed whole joints of small animals. Histochem J. 1992 Feb;24(2):115–119. doi: 10.1007/BF01082448. [DOI] [PubMed] [Google Scholar]
- Nagy E., Chalmers I. M., Baragar F. D., Friesen H. G., Berczi I. Prolactin deficiency in rheumatoid arthritis. J Rheumatol. 1991 Nov;18(11):1662–1668. [PubMed] [Google Scholar]
- O'Sullivan F. X., Fassbender H. G., Gay S., Koopman W. J. Etiopathogenesis of the rheumatoid arthritis-like disease in MRL/l mice. I. The histomorphologic basis of joint destruction. Arthritis Rheum. 1985 May;28(5):529–536. doi: 10.1002/art.1780280511. [DOI] [PubMed] [Google Scholar]
- Ostensen M., Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983 Sep;26(9):1155–1159. doi: 10.1002/art.1780260915. [DOI] [PubMed] [Google Scholar]
- Petri M., Howard D., Repke J. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991 Dec;34(12):1538–1545. doi: 10.1002/art.1780341210. [DOI] [PubMed] [Google Scholar]
- Ratkay L. G., Chowdhary R. K., Neyndorff H. C., Tonzetich J., Waterfield J. D., Levy J. G. Photodynamic therapy; a comparison with other immunomodulatory treatments of adjuvant-enhanced arthritis in MRL-lpr mice. Clin Exp Immunol. 1994 Mar;95(3):373–377. doi: 10.1111/j.1365-2249.1994.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratkay L. G., Tonzetich J., Waterfield J. D. Antibodies to extracellular matrix proteins in the sera of MRL-lpr mice. Clin Immunol Immunopathol. 1991 May;59(2):236–245. doi: 10.1016/0090-1229(91)90021-2. [DOI] [PubMed] [Google Scholar]
- Ratkay L. G., Zhang L., Tonzetich J., Waterfield J. D. Complete Freund's adjuvant induces an earlier and more severe arthritis in MRL-lpr mice. J Immunol. 1993 Nov 1;151(9):5081–5087. [PubMed] [Google Scholar]
- Rook G. A., Steele J., Brealey R., Whyte A., Isenberg D., Sumar N., Nelson J. L., Bodman K. B., Young A., Roitt I. M. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 1991 Oct;4(5):779–794. doi: 10.1016/0896-8411(91)90173-a. [DOI] [PubMed] [Google Scholar]
- Schlaghecke R., Lenhardt E., Fortkamp E., Juli E., Zeidler H. Prophylaktischer und therapeutischer Effekt der Ostrogengabe bei der Adjuvans-Arthritis. Z Rheumatol. 1989 Nov-Dec;48(6):313–316. [PubMed] [Google Scholar]
- Seggev J. S., Sunderrajan E. V., Palomo T., McKenzie W. N., Braun S. R., O'Sullivan F. X., Walker S. E. Pulmonary perivascular and interstitial inflammation in MRL/MpJ-lpr/lpr mice. III. Modulation by cyclophosphamide and sex hormones in 4- and 6-month-old animals. Clin Immunol Immunopathol. 1991 Aug;60(2):289–298. doi: 10.1016/0090-1229(91)90071-h. [DOI] [PubMed] [Google Scholar]
- Shear H. L., Wofsy D., Talal N. Effects of castration and sex hormones on immune clearance and autoimmune disease in MRL/Mp-lpr/lpr and MRL/Mp-+/+ mice. Clin Immunol Immunopathol. 1983 Mar;26(3):361–369. doi: 10.1016/0090-1229(83)90120-4. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Roman E., Silman A. J. The pill, parity, and rheumatoid arthritis. Arthritis Rheum. 1990 Jun;33(6):782–789. doi: 10.1002/art.1780330604. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Thompson S. J., Hitsumoto Y., Zhang Y. W., Rook G. A., Elson C. J. Agalactosyl IgG in pristane-induced arthritis. Pregnancy affects the incidence and severity of arthritis and the glycosylation status of IgG. Clin Exp Immunol. 1992 Sep;89(3):434–438. doi: 10.1111/j.1365-2249.1992.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urowitz M. B., Gladman D. D., Farewell V. T., Stewart J., McDonald J. Lupus and pregnancy studies. Arthritis Rheum. 1993 Oct;36(10):1392–1397. doi: 10.1002/art.1780361011. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M. Enzyme histochemical reactions in unfixed and undecalcified cryostat sections of mouse knee joints with special reference to arthritic lesions. Histochemistry. 1986;86(2):127–133. doi: 10.1007/BF00493377. [DOI] [PubMed] [Google Scholar]
- Waites G. T., Whyte A. Effect of pregnancy on collagen-induced arthritis in mice. Clin Exp Immunol. 1987 Mar;67(3):467–476. [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- van den Brink H. R., Lems W. F., van Everdingen A. A., Bijlsma J. W. Adjuvant oestrogen treatment increases bone mineral density in postmenopausal women with rheumatoid arthritis. Ann Rheum Dis. 1993 Apr;52(4):302–305. doi: 10.1136/ard.52.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]