Abstract
Publication of the NCCLS M100-S12 document in January 2002 introduced ceftriaxone and cefotaxime MIC interpretative breakpoints of ≤1 μg/ml (susceptible), 2 μg/ml (intermediate), and ≥4 μg/ml (resistant) for nonmeningeal isolates of Streptococcus pneumoniae. To estimate the effect of these breakpoint changes on clinical laboratory susceptibility testing results, nonmeningeal pneumococcal isolate (blood and respiratory) data from The Surveillance Network Database-USA, an electronic surveillance database, for the years 1996 to 2000 were collated and studied. Of 9,863 nonmeningeal isolates tested against ceftriaxone, 82.7% were susceptible, 13.2% were intermediate, and 4.1% were resistant by the M100-S11 NCCLS breakpoints (2001); by M100-S12 breakpoints, 95.9% of the isolates were susceptible, 3.1% were intermediate, and 1.0% were resistant. Of 10,777 nonmeningeal isolates tested against cefotaxime, 79.2% were susceptible, 14.3% were intermediate, and 6.5% were resistant by M100-S11 breakpoints; by M100-S12 breakpoints, 93.5% were susceptible, 4.2% were intermediate, and 2.3% were resistant. Overall, the new M100-S12 ceftriaxone and cefotaxime interpretative breakpoints for nonmeningeal isolates of S. pneumoniae decreased the number of isolates interpreted as intermediate by 10% and as resistant by 3 to 4%.
Ceftriaxone and cefotaxime MIC interpretative breakpoints for meningeal and nonmeningeal isolates of Streptococcus pneumoniae were published by the NCCLS in the M100-S12 document in January 2002 (12). Previously, a single set of MIC interpretive breakpoints (M100-S11, 2001) for both meningeal and nonmeningeal isolates was used (11). M100-S11 MIC interpretative breakpoints for ceftriaxone and cefotaxime were 0.5 μg/ml (susceptible), 1 μg/ml (intermediate), and 2 μg/ml (resistant) (11). The M100-S12 breakpoints are 0.5 μg/ml (susceptible), 1 μg/ml (intermediate), and 2 μg/ml (resistant) for meningeal isolates and 1 μg/ml (susceptible), 2 μg/ml (intermediate), and 4 μg/ml (resistant) for nonmeningeal isolates for both ceftriaxone and cefotaxime (12). The M100-S12 interpretative guidelines advise laboratories to report both meningeal and nonmeningeal interpretative criteria for pneumococcal isolates recovered from body sites other than cerebrospinal fluid; isolates from cerebrospinal fluid should be reported with meningeal interpretative criteria only (12).
The introduction of revised amoxicillin and amoxicillin-clavulanate breakpoints in M100-S10 (10) resulted in greater than twice the number of pneumococcal isolates being interpreted as intermediate to ceftriaxone (10.3%) than to amoxicillin (4.2%) or amoxicillin-clavulanate (4.6%) and greater than six times more isolates being interpreted as resistant to ceftriaxone (14.4%) than to amoxicillin (2.2%) or amoxicillin-clavulanate (1.7%) (4). Similarly, Oteo et al. reported 23.6% of isolates to be cefotaxime intermediate and 4.5% to be cefotaxime resistant compared with 5.6% being amoxicillin intermediate and 3.8% being amoxicillin resistant despite MICs at which 90% of the isolates tested were inhibited (MIC90s) being one doubling-dilution lower for cefotaxime than for amoxicillin (13). The apparent discrepancies in the activities of amoxicillin and amoxicillin-clavulante and of ceftriaxone and cefotaxime are the result of amoxicillin and amoxicillin-clavulanate breakpoints being set to reflect their exclusive use in nonmeningeal infections. In contrast, ceftriaxone and cefotaxime breakpoints were originally set based upon each agent's activity against meningeal infections only. Other investigators have also proposed revisions to penicillin breakpoints, specifically, an increase in penicillin-susceptible, -intermediate, and -resistant breakpoints to ≤1, 2, and ≥4 μg/ml, respectively, for pneumococci isolated from patients with community-acquired pneumonia and to be used in surveillance for penicillin-resistant S. pneumoniae (6).
The current study was conducted to determine the effect of one of the latest MIC interpretive breakpoint changes: ceftriaxone and cefotaxime tested against nonmeningeal isolates of S. pneumoniae. To accomplish this, ceftriaxone and cefotaxime in vitro susceptibility testing data for meningeal and nonmeningeal isolates of S. pneumoniae included in The Surveillance Network (TSN) Database-USA (Focus Technologies, Inc., Herndon, Virginia) from January 1996 to December 2000 were interpreted using both M100-S11 (11) and M100-S12 (12) breakpoints. For the purposes of this study, nonmeningeal isolates were limited to those cultured from blood and respiratory tract sources. Respiratory tract isolates included those from both the lower (sputum, bronchial washings, and tracheal aspirates) and upper (sinus, nasopharyngeal, throat, and nose specimens) respiratory tracts. Ceftriaxone MIC results were available for 251 meningeal and 9,863 nonmeningeal (45.2% from blood; 44.8% from lower respiratory tract specimens; 10.0% from upper respiratory tract specimens) isolates of S. pneumoniae. Cefotaxime MIC results were available for 383 meningeal and 10,777 nonmeningeal (47.3% from blood; 42.5% from lower respiratory tract specimens; 10.2% from upper respiratory tract specimens) isolates of S. pneumoniae.
The M100-S11 ceftriaxone and cefotaxime breakpoints have remained unchanged from 1996 and were in effect until December 2001. The nonmeningeal isolates were also stratified by their penicillin MICs for further analysis: penicillin susceptible (MIC, ≤0.06 μg/ml); penicillin intermediate (MIC, 0.12 to 1 μg/ml); and penicillin resistant (MIC, ≥2 μg/ml) (11). In addition, nonmeningeal isolates of S. pneumoniae were studied for the prevalence of associated antimicrobial resistance. Only isolates concurrently tested for susceptibility to penicillin, ceftriaxone, erythromycin, trimethoprim-sulfamethoxazole (SXT), and levofloxacin in 2000 were included in this analysis. A separate analysis of multidrug resistance (MDR) involving cefotaxime was not performed, as results were anticipated to be very similar to those for ceftriaxone. Resistance to zero, one, two, three, four, and all five antimicrobials was determined for each isolate. MDR was defined as concurrent resistance to three or more of the five antimicrobials studied. The prevalence of MDR was assessed twice for isolates, once with M100-S11 ceftriaxone breakpoints (11) and again with the M100-S12 breakpoints (12), to determine the impact of the breakpoint change on the prevalence of MDR.
TSN Database-USA, initiated in 1994 by Focus Technologies, Inc. (formerly MRL), is a queriable real-time database that electronically assimilates antimicrobial susceptibility testing and patient demographic data from a network of hospitals in the United States (17). The number of laboratories participating in TSN was 58 in 1996, 122 in 1997, 186 in 1998, 232 in 1999, and 258 in 2000. Laboratories are included in TSN based on factors such as hospital bed size, patient population, geographic location, and antimicrobial susceptibility testing methods used (17). Susceptibility testing of patient isolates is conducted onsite by each participating laboratory as a part of their routine diagnostic testing. Only data generated using Food and Drug Administration-approved testing methods with MIC results interpreted according to NCCLS recommendations are included in TSN. In addition, a series of quality control filters (i.e., critical rule sets) are used to screen susceptibility test results for patterns indicative of testing error; suspect results are removed from analysis for laboratory confirmation.
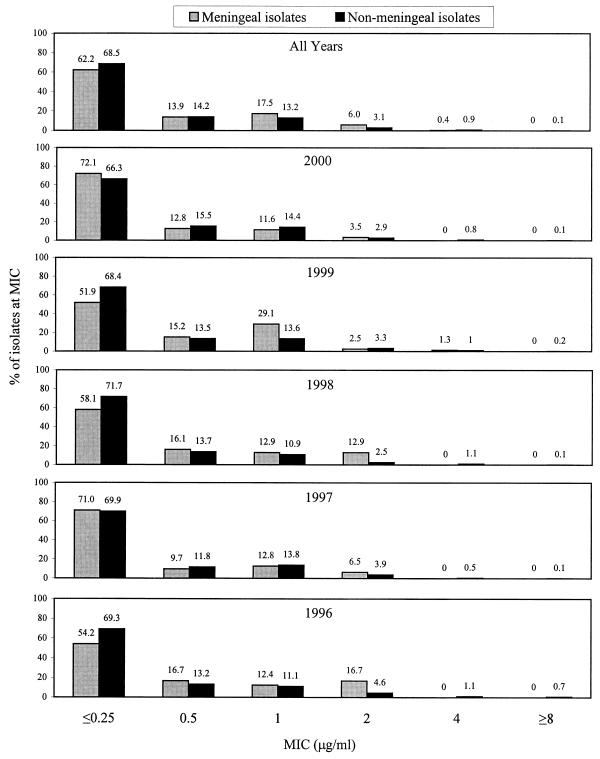
Figure 1 depicts ceftriaxone MIC distributions for meningeal and nonmeningeal isolates of S. pneumoniae and indicates that meningeal and nonmeningeal isolates were approximately equally susceptible to ceftriaxone from 1996 to 2000. The number of meningeal isolate results available for each year were limited: 20 (1996), 31 (1997), 31 (1998), 79 (1999), and 86 (2000). The number of nonmeningeal isolate ceftriaxone results available each year were 591 (1996), 836 (1997), 1,602 (1998), 3,076 (1999), and 3,758 (2000). The increasing number of isolates each year reflects increasing laboratory enrollment in TSN Database-USA from 1996 to 2000 as well as increased frequency of S. pneumoniae isolation per institution over time. Cefotaxime MIC distributions for meningeal and nonmeningeal isolates of S. pneumoniae were very similar to those for ceftriaxone (data not shown).
FIG. 1.
Ceftriaxone MIC distributions for meningeal (n = 251) and nonmeningeal (n = 9,863) isolates of S. pneumoniae; cumulative data from TSN Database-USA collected between 1996 and 2000.
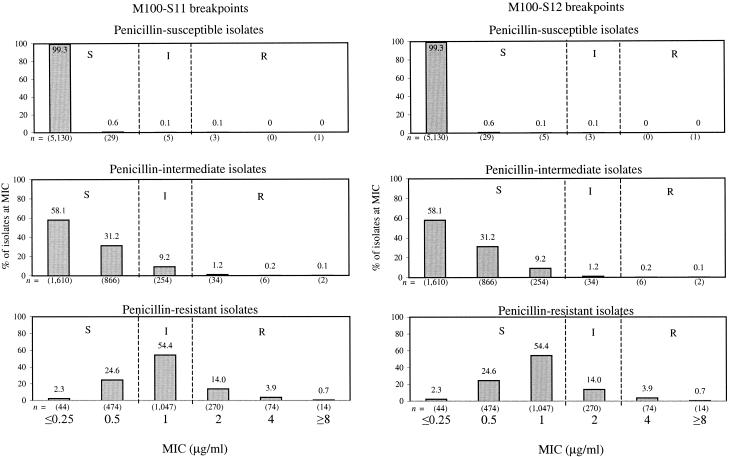
Figure 2 provides MIC distributions for ceftriaxone against nonmeningeal isolates of pneumococci that have been separated into penicillin-susceptible, penicillin-intermediate, and penicillin-resistant groups. All but 9 (0.2%) penicillin-susceptible isolates were inhibited by ceftriaxone at a concentration of 0.5 μg/ml, the M100-S11 susceptible breakpoint (11). Among penicillin-intermediate isolates, 89.3% (2,476 out of 2,772) were inhibited at 0.5 μg/ml and 98.5% (2,730 out of 2,772) were inhibited at 1 μg/ml, the M100-S12 susceptible breakpoint (12). Among penicillin-resistant isolates, 26.9% (518 out of 1,923) were inhibited at 0.5 μg/ml and 81.4% (1,565 out of 1,923) were inhibited at 1 μg/ml. Among penicillin-resistant isolates, 18.6% were ceftriaxone resistant by M100-S11 breakpoints, while only 4.6% were ceftriaxone resistant by M100-S12 breakpoints. Cefotaxime MIC distributions for penicillin-susceptible, -intermediate, and -resistant isolates were similarly affected by the application of the M100-S12 breakpoints (data not shown).
FIG. 2.
MIC distributions and interpretive breakpoints for ceftriaxone against nonmeningeal isolates of penicillin-susceptible, -intermediate, and -resistant S. pneumoniae: cumulative data from TSN Database-USA collected between 1996 and 2000. Total data for penicillin-susceptible isolates were as follows: n = 5,168; MIC50, ≤0.25 μg/ml; MIC90, ≤0.25 μg/ml; modal MIC, ≤0.25 μg/ml; MIC range, ≤0.25 to ≥8 μg/ml. Total data for penicillin-intermediate isolates were as follows: n = 2,772; MIC50, ≤0.25 μg/ml, MIC90, ≤1 μg/ml; modal MIC, ≤0.25 μg/ml; MIC range, ≤0.25 to ≥8 μg/ml. Total data for penicillin-resistant isolates were as follows: n = 1,923; MIC50, 1 μg/ml; MIC90, 2 μg/ml; modal MIC, 1 μg/ml; MIC range, ≤0.25 to ≥8 μg/ml.
Table 1 shows that applying the M100-S12 ceftriaxone breakpoints resulted in 95.9% of isolates being interpreted as susceptible, 3.1% as intermediate, and 1.0% as resistant. Among the same isolate set, using M100-S11 breakpoints resulted in 82.7% of isolates being interpreted as susceptible, 13.2% as intermediate, and 4.1% as resistant. Among the isolates tested against cefotaxime, the application of M100-S12 cefotaxime breakpoints resulted in 93.5% of isolates being interpreted as susceptible, 4.2% as intermediate, and 2.3% as resistant. Among the same isolate set, using M100-S11 breakpoints resulted in 79.2% of isolates being interpreted as susceptible, 14.3% as intermediate, and 6.5% as resistant. Table 1 also summarizes the differences in the percentages of penicillin-susceptible, -intermediate, and -resistant isolates interpreted as ceftriaxone and cefotaxime susceptible, intermediate, and resistant.
TABLE 1.
Susceptible, intermediate, and resistant interpretations of ceftriaxone and cefotaxime MICs for nonmeningeal isolates of S. pneumoniae using NCCLS M100-S11 and M100-S12 breakpointsa
| Drug and isolate type | n | MIC interpretative breakpoint
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| M100-S11b
|
M100-S12c
|
||||||||
| % S | % I | % R | % S | % I | % R | ||||
| Ceftriaxone | |||||||||
| All isolates | 9,863 | 82.7 | 13.2 | 4.1 | 95.9 | 3.1 | 1.0 | ||
| Penicillin susceptible | 5,168 | 99.8 | 0.1 | 0.1 | 99.9 | 0.1 | <0.1 | ||
| Penicillin intermediate | 2,772 | 89.3 | 9.2 | 1.5 | 98.5 | 1.2 | 0.3 | ||
| Penicillin resistant | 1,923 | 26.9 | 54.4 | 18.6 | 81.4 | 14.0 | 4.6 | ||
| Cefotaxime | |||||||||
| All isolates | 10,777 | 79.2 | 14.3 | 6.5 | 93.5 | 4.2 | 2.3 | ||
| Penicillin susceptible | 5,808 | 99.8 | 0.1 | 0.1 | 99.9 | 0.1 | <0.1 | ||
| Penicillin intermediate | 2,960 | 83.1 | 14.7 | 2.2 | 97.8 | 1.6 | 0.6 | ||
| Penicillin resistant | 2,009 | 13.7 | 55.2 | 31.2 | 68.8 | 20.0 | 11.2 | ||
Nonmeningeal isolates were composed of respiratory and blood isolates. Shown are cumulative data from TSN Database-USA that were collected from 1996 to 2000. S, susceptible; I, intermediate; R, resistant.
≤0.5 (susceptible), 1 (intermediate), and ≥2 (resistant) μg/ml (15).
Nonmeningeal breakpoints were used: ≤1 (susceptible), 2 (intermediate), and ≥4 (resistant) μg/ml (16).
The rates of MDR among the 544 isolates of S. pneumoniae tested against penicillin, ceftriaxone, erythromycin, SXT, and levofloxacin in the year 2000 were compared using M100-S11 (2 μg/ml) and M100-S12 (4 μg/ml) ceftriaxone resistance breakpoints. The rate of MDR was 17.7% using the M100-S11 breakpoint and was 16.7% using the M100-S12 breakpoint (data not shown). Rates of resistance to zero (47.7 and 52.9%), one (18.6 and 14.0%), two (16.0 and 16.3%), three (15.4 and 15.8%), four (2.3 and 1.0%), and all five (0 and 0%) antimicrobials for M100-S11 and M100-S12, respectively, were similar. Even though there was little effect on the overall prevalence of MDR, using the M100-S12 ceftriaxone resistance breakpoint, the prevalence of ceftriaxone resistance as a component of MDR decreased from 19.7% of MDR isolates (3.5% of all 544 isolates) to 8.0% of MDR isolates (1.3% of all isolates). This greater difference, compared with the overall data, arises because ceftriaxone resistance appeared predominantly in isolates resistant to three other antimicrobials. Even when the interpretation of some isolates' ceftriaxone MICs changed from resistant to intermediate, these isolates remained resistant to three other antimicrobials and thus continued to be defined as MDR.
Results from the study presented here indicate that the M100-S12 nonmeningeal breakpoints published in January 2002 (12) will significantly alter the percentages of these isolates interpreted as susceptible, intermediate, and resistant (Table 1). For example, in this study, susceptiblity to ceftriaxone among nonmeningeal isolates of S. pneumoniae increased from 82.7 to 95.9% and resistance decreased from 4.1 to 1.0% using the new breakpoints (Table 1). Similar observations were made for cefotaxime (Table 1). The marked differences in susceptible, intermediate, and resistant interpretations are attributable to the 16.4% (ceftriaxone) and 18.5% (cefotaxime) of isolates that have MICs of 1 and 2 μg/ml and whose interpretations change with application of the M100-S12 breakpoints. The M100-S12 breakpoints resulted in 98.5% of penicillin-intermediate isolates being interpreted as ceftriaxone susceptible and 97.8% as cefotaxime susceptible. The majority of penicillin-resistant isolates were also interpreted as susceptible to ceftriaxone (81.4%) and cefotaxime (68.8%) with M100-S12 breakpoints.
Previous centralized surveillance studies in the United States have reported the majority of penicillin-resistant isolates as nonsusceptible to cefotaxime (62.6%, intermediate; 31.5%, resistant) (19) and ceftriaxone (33.7 to 58.4%, intermediate; 23.2 to 65.7%, resistant) (4, 18, 19). In the current study, using M100-S11 breakpoints, 19.4% of penicillin-resistant isolates were ceftriaxone resistant and 53.5% were ceftriaxone intermediate. The observed variability in percent resistance may reflect MIC clustering close to or at intermediate and resistant breakpoints and the inherent variability in MIC testing (plus or minus one doubling dilution).
Rates of ceftriaxone resistance in the United States have been reported to be 2.9% (1997 to 1998 respiratory season) to 3.4% (1998 to 1999 respiratory season) by the TRUST centralized in vitro surveillance program (16). SENTRY has recently reported cefotaxime resistance in the U.S. (n = 8,252) to be 3.7% in respiratory isolates of S. pneumoniae from 1997 to 1999 and to have remained stable during 1997 (4% resistance), 1998 (3%), and 1999 (5%) (7). Each of the aforementioned studies interpreted ceftriaxone MICs using breakpoints of 0.5 (susceptible), 1 (intermediate), and 2 (resistant) μg/ml. In two other previous centralized in vitro surveillance studies, ceftriaxone and cefotaxime MIC distributions for S. pneumoniae were provided (18, 19). Using the ceftriaxone MIC distributions from a 1996 to 1997 U.S. respiratory season study (19), 85.0, 10.8, and 4.3% of isolates were susceptible, intermediate, and resistant, respectively, by M100-S11 breakpoints, whereas 95.8, 2.8, and 1.5% of isolates were susceptible, intermediate, and resistant, respectively, by M100-S12 breakpoints. In the same study, 87.1, 9.5, and 3.4% were susceptible, intermediate, and resistant, respectively, to cefotaxime by M100-S11 interpretations, while 96.6, 1.9, and 1.5% of isolates were susceptible, intermediate, and resistant, respectively, by M100-S12 breakpoints. According to a ceftriaxone MIC distribution in a study conducted during the 1997 to 1998 respiratory season in the U.S. (18), 87.9, 9.0, and 3.2% of isolates were susceptible, intermediate, and resistant, respectively, to ceftriaxone by M100-S11 interpretations, while 96.9, 1.9, and 1.3% of isolates were susceptible, intermediate, and resistant, respectively, by M100-S12 breakpoints. Cefotaxime data were not available in this second study.
Even though ceftriaxone and cefotaxime have identical MIC interpretative breakpoints, pharmacokinetic and pharmacodynamic differences exist between these two parenteral cephalosporins. These differences are important, as β-lactam levels in serum or at the site of infection need to exceed the MIC for isolates for >40% of the dosing interval for maximal bacteriologic efficacy and patient survival (2). This relationship does not differ for penicillin-susceptible and penicillin-resistant strains of S. pneumoniae (1). The speed of drug clearance from the body is an important determinant of the duration of time that serum levels exceed the MIC of susceptible pathogens: the half-life of broad-spectrum cephalosporins in healthy young volunteers varies from about 1 h for cefotaxime to approximately 8 h for ceftriaxone (3). For cefotaxime dosed at 1 g every 8 h, the time serum levels remain above the MIC is 63 to 87% of the dosing interval for penicillin-intermediate isolates and 52 to 63% of the dosing interval for penicillin-resistant isolates (2). For ceftriaxone dosed at 1 g every 24 h, time above the MIC is 76 to 100% of the dosing interval for penicillin-intermediate isolates and 48 to 76% of the dosing interval for penicillin-resistant isolates (2). This difference in parenteral cephalosporin pharmacokinetics and pharmacodynamics is addressed in a comment appearing in the M100-S12 document (12). The comment indicates that use of interpretative criteria for cefotaxime and nonmeningeal isolates requires doses appropriate for serious pneumococcal infections (e.g., at least 1 g [adults] or 50 mg [children] per kg of body weight every 8 h or more frequently) (12). The importance of pharmacokinetic and pharmacodynamic parameters is supported by both animal and clinical studies (2). Craig has validated a pharmacokinetic/pharmacodynamic breakpoint for ceftriaxone in nonmeningeal infections of 2 μg/ml (2). A pharmacokinetic/pharmacodynamic breakpoint is defined as the antimicrobial concentration in serum that is maintained for >40% of the dosing interval (2).
The clinical impact of pneumococcal resistance varies with the site of infection, the degree of antimicrobial penetration to the site of infection, and the ability of the immune response to clear the infection (8). Pneumococcal resistance can lead to treatment failures for patients with meningitis and acute otitis media. The impact of pneumococcal resistance on the treatment of pneumonia has been more difficult to determine (5, 8). There exists evidence suggesting that no variance in outcomes of pneumonia is attributable to differences in the penicillin susceptibility of pneumococcal isolates (14, 15), although there is also recent evidence that increased morbidity and mortality are associated with high-level β-lactam resistance (5, 9, 20). Treatment with parenteral broad-spectrum cephalosporins in a large number of patients with severe pneumococcal pneumonia has been demonstrated not to result in any difference in outcome for patients infected with penicillin-resistant strains compared with those infected with penicillin-susceptible strains (14; R. Pallares, O. Capdevila, J. Linares, I. Grau, H. Onaga, F. Tubua, and F. Gudiol, Program Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-903, 2001).
Tracking the prevalence of MDR pneumococci is important, as the therapeutic choices for such organisms can become quite limited. The near doubling in the percentage of isolates exhibiting MDR phenotypes in the United States between 1997 and 1998 (5.9%) and 1998 and 1999 (11%) is alarming (16). Similar results were reported from a study of invasive pneumococcal infections among patients in the United States from 1995 to 1998 in which MDR increased from 9 to 14% (21). In the current study, using the M100-S12 ceftriaxone resistance breakpoint to recalculate the rate of MDR among nonmeningeal isolates of S. pneumoniae in the year 2000 resulted in only a 1.0% decrease in MDR compared with the rate using the M100-S11 ceftriaxone resistance breakpoint. Even though there was little impact on the overall prevalence of MDR, application of the M100-S12 ceftriaxone resistance breakpoint resulted in ceftriaxone resistance as a component of MDR decreasing from 19.7% of MDR isolates (3.5% of all isolates) to 8.0% of MDR isolates (1.3% of all isolates). Clearly, monitoring and tracking MDR phenotypes needs to be a key component of pneumococcal surveillance initiatives.
Among collections of approximately 10,000 nonmeningeal isolates of S. pneumoniae tested against ceftriaxone and cefotaxime in clinical laboratories in the United States between 1996 and 2000, the M100-S12 breakpoints lowered the percentages of isolates that were interpreted as intermediate and resistant to ceftriaxone and cefotaxime. The largest difference in susceptibility to ceftriaxone and cefotaxime was observed when M100-S12 breakpoint changes were applied to penicillin-resistant isolates: 81.4% (ceftriaxone) and 68.8% (cefotaxime) of isolates were interpreted as susceptible by M100-S12, compared with 26.9% (ceftriaxone) and 13.7% (cefotaxime) interpreted as susceptible using M100-S11. The publication and use of M100-S12 breakpoints to interpret ceftriaxone and cefotaxime MICs for nonmeningeal isolates of S. pneumoniae will more accurately reflect the clinical activities of these agents for nonmeningeal infections where a broad-spectrum cephalosporin may be indicated. In addition, M100-S12 breakpoints for nonmeningeal isolates will bring the in vitro activities of ceftriaxone and cefotaxime into closer agreement with oral β-lactams, such as amoxicillin and amoxicillin-clavulanate, that have also recently undergone breakpoint changes to reflect their exclusive use in nonmeningeal infections.
Acknowledgments
We thank the participating institutions in TSN Database-USA who make data collection possible and Roche Laboratories, Inc., who financially supported the study.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 4.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. L. Coffman, and A. B. Brueggeman. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feikin, D. R., A. Schuchat, M. Kolczak, N. L. Barrett, L. H. Harrison, L. Lefkowitz, A. McGeer, M. M. Farley, D. J. Vugia, C. Lexau, K. R. Stefonek, J. E. Patterson, and J. H. Jorgensen. 2000. Mortality from invasive pneumoccal pneumonia in the era of antibiotic resistance, 1995-1997. Am. J. Public Health 90:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 7.Hoban, D. J., G. V. Doern, A. C. Fluit, M. Roussel-Delvallez, and R. N. Jones. 2001. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S81-S93. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, S. L., and E. O. Mason, Jr. 1998. Management of infections due to antibiotic resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 11:628-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metlay, J. P., J. Hofmann, M. S. Cetron, M. J. Fine, M. M. Farley, C. Whitney, and R. F. Breiman. 2000. Impact of penicillin susceptibility on medical outcomes for adult patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 30:520-528. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing; tenth informational supplement, vol. 20, no. 1. M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing; eleventh informational supplement, vol. 21, no. 1. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; twelvth informational supplement, vol. 22, no. 1. M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Oteo, J., J. I. Alós, and J. L. Gómez-Garcés. 2001. Antimicrobial resistance of Streptococcus pneumoniae isolates in 1999 and 2000 in Madrid, Spain: a multicentre surveillance study. J. Antimicrob. Chemother. 47:215-218. [DOI] [PubMed] [Google Scholar]
- 14.Pallares, R., J. Liñares, M. Vadillo, C. Cabellos, F. Manresa, P. F. Vradrich, R. Martin, and F. Gudiol. 1995. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N. Engl. J. Med. 333:474-480. [DOI] [PubMed] [Google Scholar]
- 15.Rosón, B., J. Carratalà, F. Tubua, J. Dorca, J. Liñares, R. Pallares, F. Manresa, and F. Gudiol. 2001. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb. Drug Resist. 7:85-96. [DOI] [PubMed] [Google Scholar]
- 16.Sahm, D. F., J. A. Karlowsky, L. J. Kelly, I. A. Critchley, M. E. Jones, C. Thornsberry, Y. Mauriz, and J. Kahn. 2001. Need for annual surveillance of antimicrobial resistance in Streptococcus pneumoniae in the United States: 2-year longitudinal analysis. Antimicrob. Agents Chemother. 45:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahm, D. F., M. K. Marsilio, and G. Piazza. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with The Surveillance Network Database—USA. Clin. Infect. Dis. 29:259-263. [DOI] [PubMed] [Google Scholar]
- 18.Thornsberry, C., M. E. Jones, M. L. Hickey, Y. Mauriz, J. Kahn, and D. F. Sahm. 1999. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997-1998. J. Antimicrob. Chemother. 44:749-759. [DOI] [PubMed] [Google Scholar]
- 19.Thornsberry, C., P. T. Oglivie, H. P. Holley, Jr., and D. F. Sahm. 1999. Survey of susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates to 26 antimicrobial agents: a prospective U.S. study. Antimicrob. Agents Chemother. 43:2612-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turrett, G. S., S. Blum, B. A. Fasal, J. E. Justman, and E. E. Telzak. 1999. Penicillin resistance and the predictors of mortality in pneumococcal bacteremia in a population with high HIV seroprevalence. Clin. Infect. Dis. 29:321-327. [DOI] [PubMed] [Google Scholar]
- 21.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]