Abstract
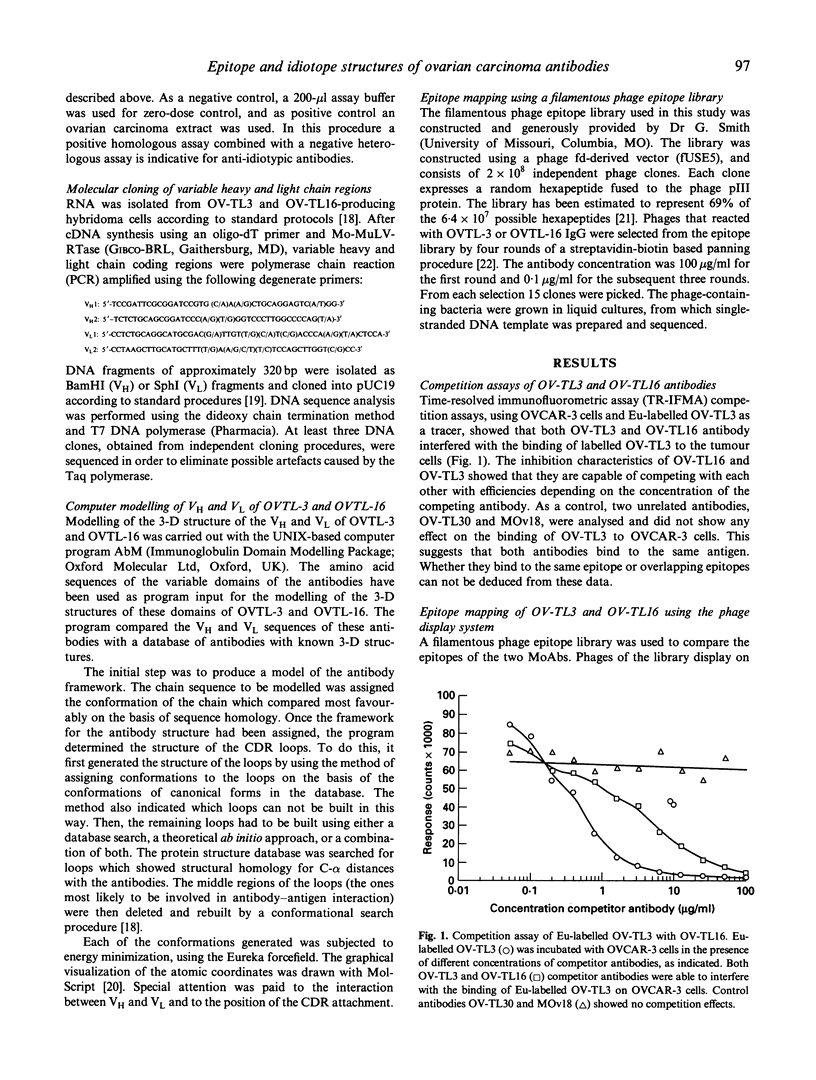
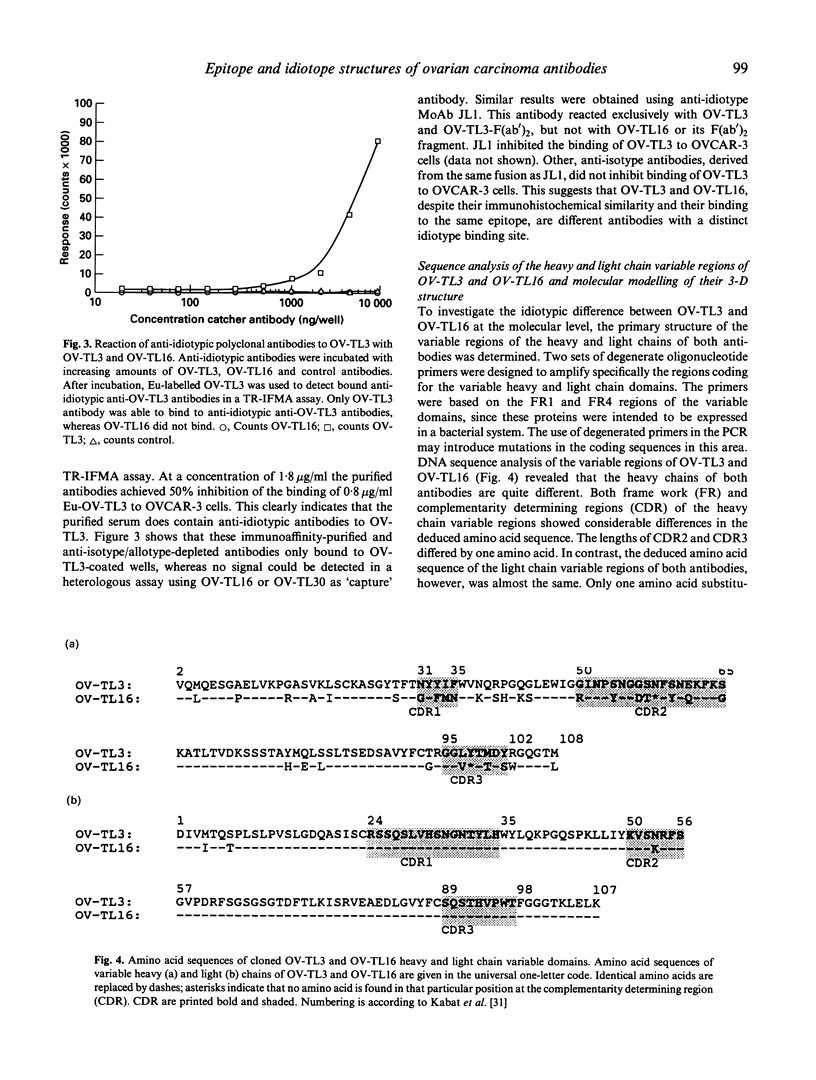
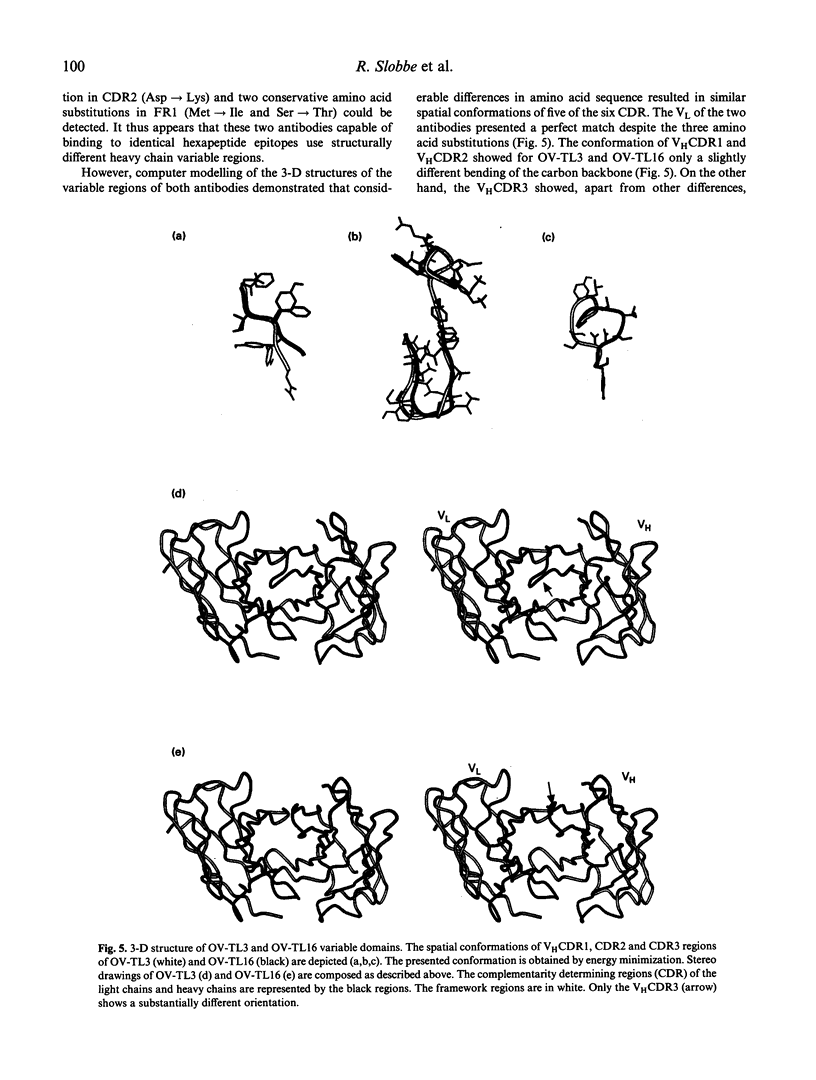
Two MoAbs, independently raised against ovarian carcinoma cells and referred to as OV-TL3 and OV-TL16, display an identical reaction pattern with a membrane-associated protein in both normal and malignant ovarian cells. Also, a similar binding affinity constant and a similar number of binding sites per cell indicate that both MoAbs bind to the same antigen. Competition assays reveal that OV-TL16 is able to compete with OV-TL3 for binding to OVCAR-3 cells. Epitope mapping using a filamentous phage hexapeptide epitope library showed that both MoAbs are able to select identical phages, suggesting that their epitopes are identical or at least overlapping. However, purified polyclonal and monoclonal anti-idiotypic antibodies directed against OV-TL3 failed to recognize the OV-TL16 idiotype, indicating that the structure of the antigen-binding regions of both antibodies is distinct. This was corroborated by molecular cloning and sequencing of the variable heavy (VH) and light (VL) chain immunoglobulin regions of both MoAbs. The VH regions of both antibodies were found to be distinct, whereas the VL regions are almost identical. Computer modelling of the idiotypes suggests that the complementarity determining regions (CDR), with the exception of VHCDR3, have (almost) identical spatial configurations. Our data indicate that, although structurally different in their VH regions, OV-TL3 and OV-TL16 are able to bind to identical epitopic regions on the antigen, because differences in primary structure do not exclude the formation of sufficient and similar spatial structures for the interaction with an epitope.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akolkar P. N., Sikder S. K., Bhattacharya S. B., Liao J., Gruezo F., Morrison S. L., Kabat E. A. Different VL and VH germ-line genes are used to produce similar combining sites with specificity for alpha(1----6)dextrans. J Immunol. 1987 Jun 15;138(12):4472–4479. [PubMed] [Google Scholar]
- Andria M. L., Levy S., Benjamini E. Diverse VH and VL genes are used to produce antibodies against a defined protein epitope. J Immunol. 1990 Apr 1;144(7):2614–2619. [PubMed] [Google Scholar]
- Boerman O. C., Makkink W. K., Thomas C. M., Hanselaar A. G., Yedema C. A., Kenemans P., Poels L. G. Monoclonal antibodies that discriminate between human ovarian carcinomas and benign ovarian tumours. Eur J Cancer. 1990 Feb;26(2):117–127. doi: 10.1016/0277-5379(90)90293-3. [DOI] [PubMed] [Google Scholar]
- Boerman O., Makkink K., Massuger L., Thomas C., Kenemans P., Hanselaar T., Poels L. Monoclonal antibodies against ovarian carcinoma-associated antigens, raised by immunization with cyst fluids. Anticancer Res. 1989 May-Jun;9(3):551–558. [PubMed] [Google Scholar]
- Boerman O., Massuger L., Makkink K., Thomas C., Kenemans P., Poels L. Comparative in vitro binding characteristics and biodistribution in tumor-bearing athymic mice of anti-ovarian carcinoma monoclonal antibodies. Anticancer Res. 1990 Sep-Oct;10(5A):1289–1295. [PubMed] [Google Scholar]
- Bruccoleri R. E., Haber E., Novotný J. Structure of antibody hypervariable loops reproduced by a conformational search algorithm. Nature. 1988 Oct 6;335(6190):564–568. doi: 10.1038/335564a0. [DOI] [PubMed] [Google Scholar]
- Böttger V., Lane E. B. A monoclonal antibody epitope on keratin 8 identified using a phage peptide library. J Mol Biol. 1994 Jan 7;235(1):61–67. doi: 10.1016/s0022-2836(05)80013-0. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Freemont P. S., Foulkes W., Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992 Oct 1;52(19):5416–5420. [PubMed] [Google Scholar]
- Christian R. B., Zuckermann R. N., Kerr J. M., Wang L., Malcolm B. A. Simplified methods for construction, assessment and rapid screening of peptide libraries in bacteriophage. J Mol Biol. 1992 Oct 5;227(3):711–718. doi: 10.1016/0022-2836(92)90219-a. [DOI] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Hemmilä I., Dakubu S., Mukkala V. M., Siitari H., Lövgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984 Mar;137(2):335–343. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massuger L. F., Claessens R. A., Kenemans P., Verheijen R. H., Boerman O. C., Meeuwis A. P., Schijf C. P., Buijs W. C., Hanselaar T. G., Corstens F. H. Kinetics and biodistribution in relation to tumour detection with 111In-labelled OV-TL 3 F(ab')2 in patients with ovarian cancer. Nucl Med Commun. 1991 Jul;12(7):593–609. doi: 10.1097/00006231-199107000-00004. [DOI] [PubMed] [Google Scholar]
- Massuger L. F., Kenemans P., Claessens R. A., Verheijen R. H., Schijf C. P., Strijk S. P., Poels L. G., van Hoesel R. G., Corstens F. H. Immunoscintigraphy of ovarian cancer with indium-111-labeled OV-TL 3 F(ab')2 monoclonal antibody. J Nucl Med. 1990 Nov;31(11):1802–1810. [PubMed] [Google Scholar]
- Massuger L. F., Thomas C. M., Segers M. F., Corstens F. H., Verheijen R. H., Kenemans P., Poels L. G. Specific and nonspecific immunoassays to detect HAMA after administration of indium-111-labeled OV-TL 3 F(ab')2 monoclonal antibody to patients with ovarian cancer. J Nucl Med. 1992 Nov;33(11):1958–1963. [PubMed] [Google Scholar]
- Miotti S., Canevari S., Ménard S., Mezzanzanica D., Porro G., Pupa S. M., Regazzoni M., Tagliabue E., Colnaghi M. I. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987 Mar 15;39(3):297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- Nässander U. K., Steerenberg P. A., Poppe H., Storm G., Poels L. G., De Jong W. H., Crommelin D. J. In vivo targeting of OV-TL 3 immunoliposomes to ascitic ovarian carcinoma cells (OVCAR-3) in athymic nude mice. Cancer Res. 1992 Feb 1;52(3):646–653. [PubMed] [Google Scholar]
- Parmley S. F., Smith G. P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988 Dec 20;73(2):305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Poels L. G., Peters D., van Megen Y., Vooijs G. P., Verheyen R. N., Willemen A., van Niekerk C. C., Jap P. H., Mungyer G., Kenemans P. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986 May;76(5):781–791. [PubMed] [Google Scholar]
- Rini J. M., Schulze-Gahmen U., Wilson I. A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992 Feb 21;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Soini E., Kojola H. Time-resolved fluorometer for lanthanide chelates--a new generation of nonisotopic immunoassays. Clin Chem. 1983 Jan;29(1):65–68. [PubMed] [Google Scholar]
- Stark S. E., Caton A. J. Antibodies that are specific for a single amino acid interchange in a protein epitope use structurally distinct variable regions. J Exp Med. 1991 Sep 1;174(3):613–624. doi: 10.1084/jem.174.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen C. W., Lane D. P. Mutant conformation of p53. Precise epitope mapping using a filamentous phage epitope library. J Mol Biol. 1992 Jun 5;225(3):577–583. doi: 10.1016/0022-2836(92)90386-x. [DOI] [PubMed] [Google Scholar]
- Van Niekerk C. C., Ramaekers F. C., Hanselaar A. G., Aldeweireldt J., Poels L. G. Changes in expression of differentiation markers between normal ovarian cells and derived tumors. Am J Pathol. 1993 Jan;142(1):157–177. [PMC free article] [PubMed] [Google Scholar]


