Abstract
Acinetobacter isolates from eight subjects with community-acquired Acinetobacter pneumonia (CAAP), a major cause of fatal community-acquired pneumonia in tropical Australia, were phenotypically and genotypically confirmed by pulsed-field gel electrophoresis analysis to be broadly diverse Acinetobacter baumannii strains. Wet-season throat carriage of A. baumannii was found in 10% of community residents with excess levels of alcohol consumption, the major at-risk group for CAAP.
Acinetobacter species are a major cause of severe community-acquired pneumonia in tropical northern Australia, accounting for 10% of cases and 20% of deaths from bacteremic community-acquired pneumonia (1). On the basis of traditional identification methods and because of the severity of clinical disease, community-acquired Acinetobacter pneumonia (CAAP) has been thought to be due to Acinetobacter baumannii, but descriptions to date have not used modern taxonomy and the species involved remain undefined (7). We therefore undertook a study for the phenotypic and genotypic identification of Acinetobacter isolates from subjects with CAAP.
A further unresolved issue is the environmental source and human reservoir of those Acinetobacter spp. responsible for severe bacteremic disease, both community acquired and nosocomial (3, 7, 8). While A. baumannii is a rare colonizer (0.5%) of human skin in temperate climates (8), skin carriage is more common in tropical environments, being isolated in 3.8% of community subjects during the hot, humid season in Hong Kong (3). Throat carriage of A. baumannii by community subjects was not found in Germany and was not defined in Hong Kong (3, 8). Excess alcohol consumption (6) is the major risk factor for CAAP in northern Australia (1). Because microaspiration of pharyngeal organisms is postulated to precede CAAP in those with alcoholism and because of the predominance of CAAP during the wet season, we also undertook a preliminary investigation of wet-season throat carriage of Acinetobacter species in community subjects with excess levels of alcohol consumption.
We studied Acinetobacter isolates from two groups of people. The first comprised eight patients with CAAP, as defined previously (1), with CAAP being fatal in five patients and nonfatal in three patients. Six patients were hospitalized in Darwin or Gove in tropical northern Australia, and two patients were hospitalized in Alice Springs in central Australia. For seven of these eight patients, isolates were from pretreatment admission blood cultures (and, from one of these patients, sputum); for the eighth patient, the isolate was from an admission sputum specimen only (the case was fatal, the subject had multiple risk factors for CAAP, and no pretreatment blood cultures were done). The second group comprised 20 community subjects attending the Emergency Department of Royal Darwin Hospital in March and April 2001 (the end of the tropical Australian wet season) with nonrespiratory presentations and a history of excess levels of alcohol consumption, defined as alcohol intake of greater than 6 standard drinks/day (6). This group had no recent hospitalizations. Throat swabs were obtained with informed consent and were cultured on horse blood agar containing 5 mg of vancomycin per liter, 10 mg of cefazolin per liter, and 4% glucose at 35°C in air for 48 h. The studies were approved by the Health Research Ethics Committee of Territory Health Services and the Menzies School of Health Research.
Identification at the genus level was confirmed by the transformation assay of Juni (5). Phenotypic identification was performed by use of the simplified identification system of Bouvet and Grimont (2), including growth in tryptic soy broth at 37, 41, and 44°C. Species confirmation was undertaken by amplified ribosomal DNA restriction analysis (ARDRA), as described previously (10). The Acinetobacter isolates were further characterized by analysis of genomic DNA by pulsed-field gel electrophoresis (PFGE) with use of the restriction enzyme ApaI, as described previously (9).
All nine isolates from the eight patients with CAAP, including those from the five patients with fatal cases of CAAP, were confirmed by phenotypic analysis and ARDRA to be A. baumannii (DNA group 2). Two additional significant nosocomial bacteremic isolates (including one from a patient with a fatal case of CAAP) were also identified as A. baumannii. An additional two community blood culture isolates, including one from a patient with influenza pneumonia, were identified by ARDRA as Acinetobacter spp. that did not belong to the A. calcoaceticus-A. baumannii complex. These isolates were considered contaminants on clinical grounds. This finding supports the recommendation to identify Acinetobacter isolates from clinically relevant sources to the species level to assist clinicians in evaluating their clinical significance (7).
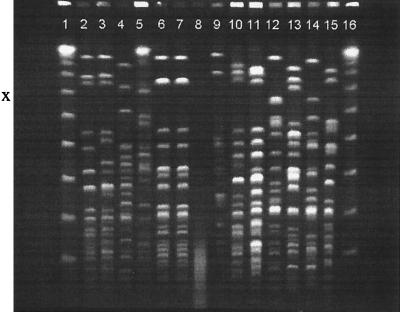
Our results therefore confirm that the fulminant CAAP seen in tropical Australia is caused by A. baumannii. PFGE analysis demonstrated broad strain diversity among the CAAP isolates, with isolates from each patient exhibiting a unique fingerprint pattern (Fig. 1). For the single patient for whom both blood and sputum underwent PFGE analysis, strain identity was demonstrated (Fig. 1, lanes 6 and 7), the first definitive proof of community-acquired bacteremic A. baumannii pneumonia.
FIG. 1.
Fingerprint patterns of A. baumannii genomic DNA obtained by PFGE after restriction with ApaI from blood and sputum isolates from patients with CAAP and from isolates from asymptomatic community residents with throat carriage and excess levels of alcohol consumption. Lanes 1 and 16, molecular size marker; lanes 3, 5, and 8 to 12, isolates from patients with CAAP; lanes 6 and 7, sputum and blood isolates from the same patient with CAAP; lanes 2 and 4, isolates that caused nosocomial A. baumannii bacteremia; lanes 13 to 15, isolates from asymptomatic community residents with throat carriage and excess levels of alcohol consumption.
Among the patients with CAAP, it is noteworthy that we did not find other members of the A. calcoaceticus-A. baumannii complex, in particular, isolates of DNA groups 3 and 13TU. In tropical Hong Kong, Houang et al. (4) found isolates of DNA group 3 to be the most common cause of Acinetobacter bacteremia in a tertiary hospital series and isolates of DNA group 13TU to be the most common respiratory tract isolates (4). However, the proportion of these infections that were community acquired or nosocomial was not defined. In temperate regions, both A. baumannii and isolates of DNA group 13TU are common and clinically important causes of nosocomial bacteremia, but CAAP is extremely rare.
The majority of cases of CAAP in tropical northern Australia occur in the wet season in subjects with excess levels of alcohol consumption (1), a group at risk of aspiration of pharyngeal bacteria. In our small pilot study of community throat colonization, Acinetobacter spp. were isolated from wet-season throat swabs in 2 of 20 (10%) subjects with excess levels of alcohol consumption. Both were identified phenotypically and genotypically as A. baumannii, the causative organism for fulminant CAAP in our region. An additional throat isolate of A. baumannii was cultured from a throat swab taken from a survivor of alcohol-associated bacteremic CAAP during attendance at an outpatient clinic during the dry season and 2 years after CAAP. Although the findings are clearly preliminary, they are consistent with the hypothesis that CAAP occurs following microaspiration of A. baumannii in those with preceding throat colonization with these species. Larger prospective seasonal studies of throat colonization in those with and without excess levels of alcohol consumption are required for definitive analysis.
Acknowledgments
We thank Danuta Stefanik, Jim Wells, Daniel Gal, and Pathology Department colleagues in Northern Territory hospitals for expert technical assistance, in particular, Kay Withnall, Julie Gaggin, and Fran Morey; Julian Elliott and Alex Brown for assistance with case identification; Bryan Bell for media development; Susan Jacups for database assistance; and emergency department colleagues for assistance with throat carriage studies.
REFERENCES
- 1.Anstey, N. M., B. J. Currie, and K. M. Withnall. 1992. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin. Infect. Dis. 14:83-91. [DOI] [PubMed] [Google Scholar]
- 2.Bouvet, P. J., and P. A. Grimont. 1987. Identification and biotyping of clinical isolates of Acinetobacter. Ann. Inst. Pasteur Microbiol. 138:569-578. [DOI] [PubMed] [Google Scholar]
- 3.Chu, Y. W., C. M. Leung, E. T. Houang, K. C. Ng, C. B. Leung, H. Y. Leung, and A. F. Cheng. 1999. Skin carriage of acinetobacters in Hong Kong. J. Clin. Microbiol. 37:2962-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houang, E. T., Y. W. Chu, C. M. Leung, K. Y. Chu, J. Berlau, K. C. Ng, and A. F. Cheng. 2001. Epidemiology and infection control implications of Acinetobacter spp. in Hong Kong. J. Clin. Microbiol. 39:228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juni, E. 1972. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J. Bacteriol. 112:917-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health and Medical Research Council. 2001. Australian alcohol guidelines: health benefits and risks. National Health and Medical Research Council, Canberra, Australia.
- 7.Seifert, H. 1999. Acinetobacter bacteremia in the tropics. Clin. Infect. Dis. 28:31-32. [DOI] [PubMed] [Google Scholar]
- 8.Seifert, H., L. Dijkshoorn, P. Gerner-Smidt, N. Pelzer, I. Tjernberg, and M. Vaneechoutte. 1997. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J. Clin. Microbiol. 35:2819-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert, H., A. Schulze, R. Baginski, and G. Pulverer. 1994. Plasmid DNA fingerprinting of Acinetobacter species other than Acinetobacter baumannii. J. Clin. Microbiol. 32:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaneechoutte, M., L. Dijkshoorn, I. Tjernberg, A. Elaichouni, P. de Vos, G. Claeys, and G. Verschraegen. 1995. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 33:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]