Abstract
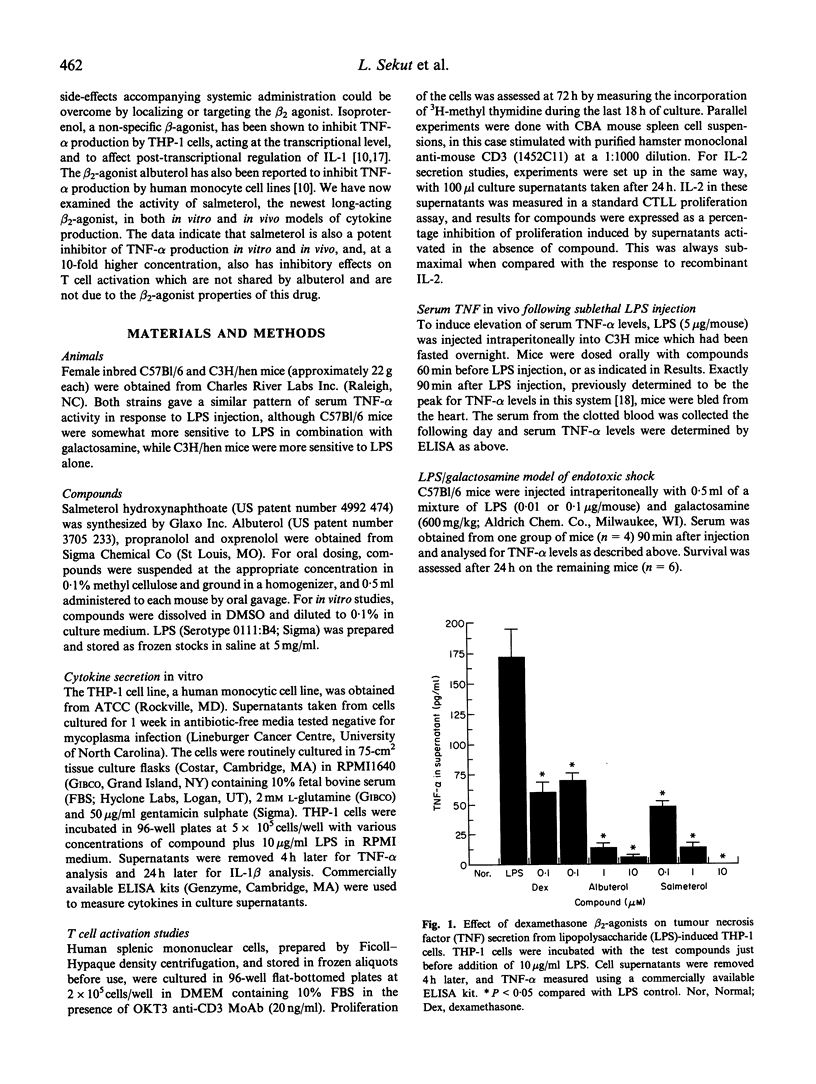
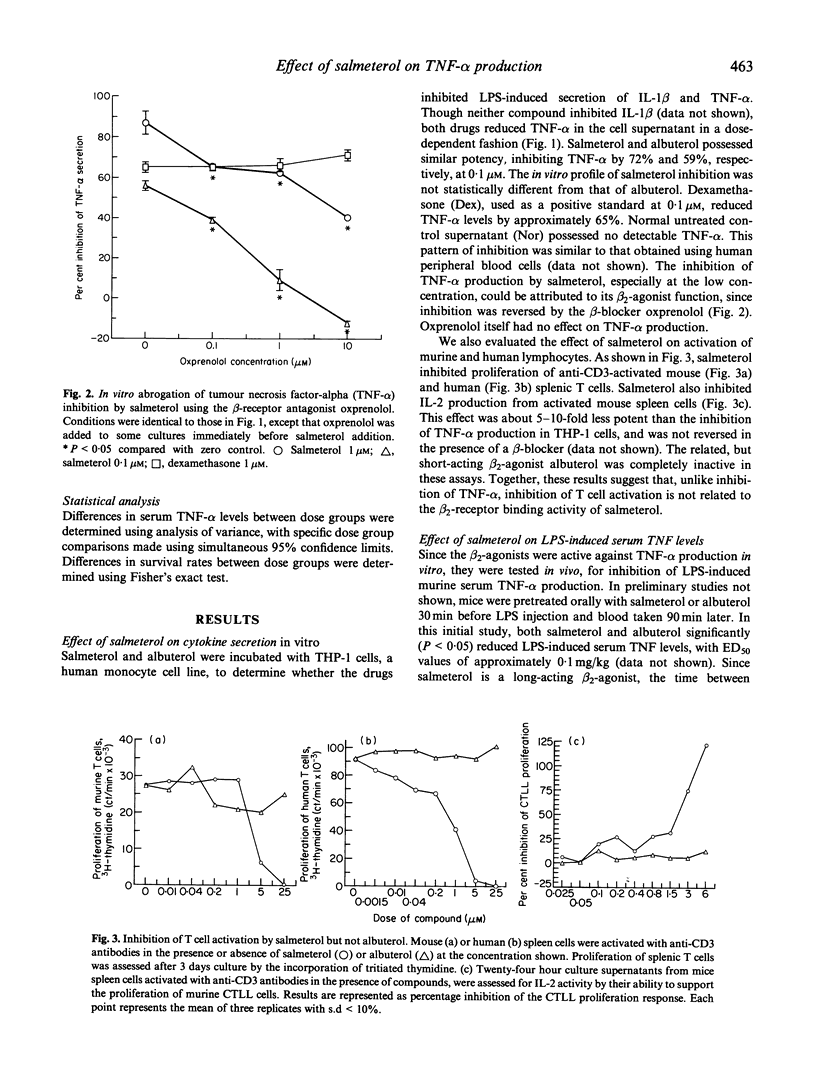
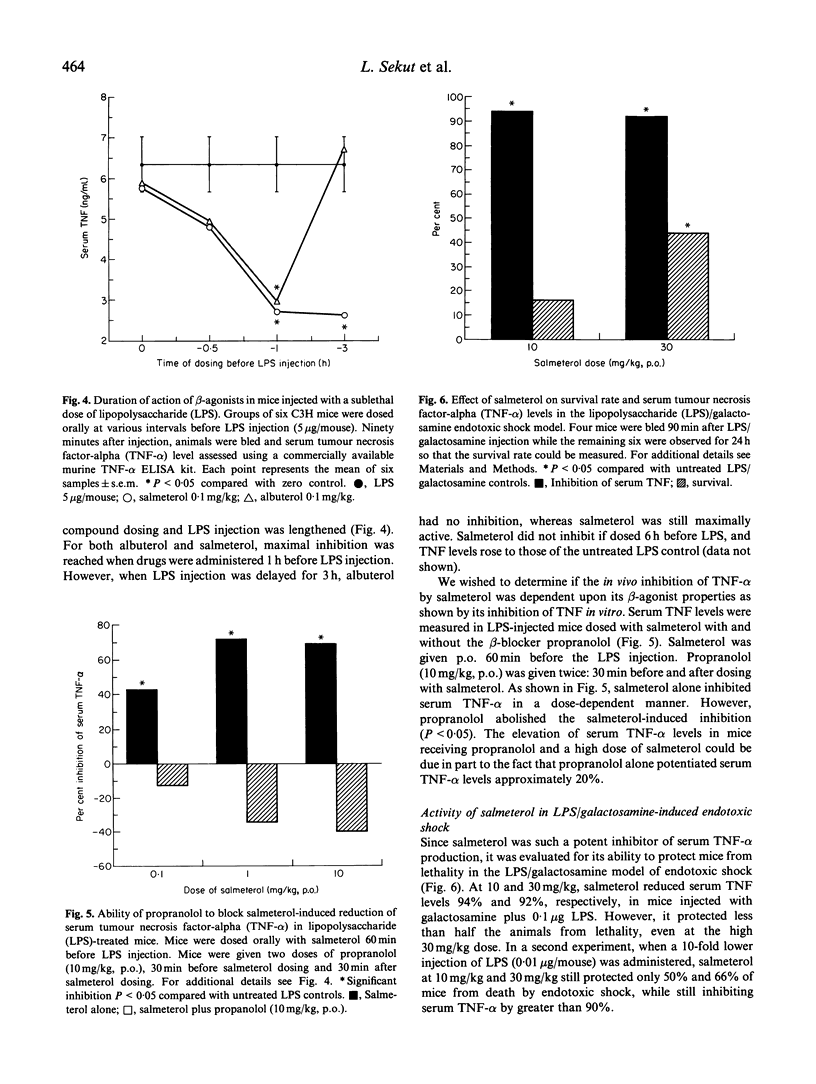
Elevation of intracellular cAMP levels has been shown previously to inhibit cytokine secretion by various cell types in vitro. Since salmeterol is a beta 2-agonist which activates adenylate cyclase, its ability to inhibit cytokine production was evaluated. Though salmeterol, and the related drug albuterol, did not inhibit IL-1 beta production in vitro, both drugs did inhibit tumour necrosis factor-alpha (TNF-alpha) secretion by lipopolysaccharide (LPS)-activated THP-1 cells with similar IC50s of approximately 0.1 microM. This inhibition was effectively reversed by the beta 2-antagonist oxprenolol, indicating that the inhibition was mediated through the beta 2-adrenergic receptor. A strikingly different reactivity profile was seen with T cells. Salmeterol was able to inhibit the activation of both mouse and human T cells, as measured by proliferation and IL-2 secretion in response to anti-CD3 antibody, whereas albuterol was completely inactive in these assays. This T cell inhibition by salmeterol was about 10-fold less potent than that for TNF-alpha production, and was not reversed by a beta 2-antagonist, indicating that a different mechanism was involved in the effect of salmeterol on T cells. Paralleling the TNF-alpha inhibitory activity in vitro, oral dosing of salmeterol and albuterol inhibited LPS-induced increase in murine serum TNF level in vivo, with ED50s of approximately 0.1 mg/kg. This inhibition could be abrogated by dosing orally with the beta-blocker propranolol. The long-acting pharmacological profile of salmeterol was apparent in that it maintained its efficacy for 3 h, while albuterol had a much shorter duration of action. Salmeterol also had some protective effects in the galactosamine/LPS model of endotoxic shock, which is dependent upon TNF-alpha production. Though salmeterol inhibited serum TNF-alpha levels by up to 94% in this assay, it protected less than 50% of the animals from the lethal effects of the LPS/galactosamine mixture. This observation suggests that functional levels of TNF-alpha localized in tissues may not be accurately reflected by serum levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball D. I., Brittain R. T., Coleman R. A., Denyer L. H., Jack D., Johnson M., Lunts L. H., Nials A. T., Sheldrick K. E., Skidmore I. F. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991 Nov;104(3):665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B. E., Jackson J. V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993 Apr;61(4):1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini F., Bruni A. Role of a serum phospholipase A1 in the phosphatidylserine-induced T cell inhibition. FEBS Lett. 1993 Jan 18;316(1):1–4. doi: 10.1016/0014-5793(93)81724-e. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Maini R. N., Feldmann M. TNF alpha--a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992 May;31(5):293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Dallman M. F., Helms C., Levine J. D. Epinephrine exacerbates arthritis by an action at presynaptic B2-adrenoceptors. Neuroscience. 1990;34(2):521–523. doi: 10.1016/0306-4522(90)90160-6. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Chan A. K., Helms C., Basbaum A. I., Levine J. D. Increasing sympathetic nerve terminal-dependent plasma extravasation correlates with decreased arthritic joint injury in rats. Neuroscience. 1991;40(1):185–189. doi: 10.1016/0306-4522(91)90184-p. [DOI] [PubMed] [Google Scholar]
- Goto M., Griffin A. J., Chiemmongkoltip P., Ohya N. Beta-adrenergic drug therapy in newborn canine endotoxic shock. Circ Shock. 1990 Oct;32(2):123–132. [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Piccoli D. S., Hamilton J. A. Control by IFN-gamma and PGE2 of TNF alpha and IL-1 production by human monocytes. Immunology. 1989 Mar;66(3):376–383. [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Hetier E., Ayala J., Bousseau A., Prochiantz A. Modulation of interleukin-1 and tumor necrosis factor expression by beta-adrenergic agonists in mouse ameboid microglial cells. Exp Brain Res. 1991;86(2):407–413. doi: 10.1007/BF00228965. [DOI] [PubMed] [Google Scholar]
- Johnson M. Salmeterol: a novel drug for the treatment of asthma. Agents Actions Suppl. 1991;34:79–95. [PubMed] [Google Scholar]
- Jones S. B., Romano F. D. Myocardial beta adrenergic receptor coupling to adenylate cyclase during developing septic shock. Circ Shock. 1990 Jan;30(1):51–61. [PubMed] [Google Scholar]
- Joyner W. L., Svensjö E., Arfors K. E. Simultaneous measurements of macromolecular leakage and arteriolar blood flow as altered by PGE1 and beta 2-receptor stimulant in the hamster cheek pouch. Microvasc Res. 1979 Nov;18(3):301–310. doi: 10.1016/0026-2862(79)90038-4. [DOI] [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Wiggins R. C., Chensue S. W., Larrick J. Regulation of macrophage tumor necrosis factor production by prostaglandin E2. Biochem Biophys Res Commun. 1986 May 29;137(1):404–410. doi: 10.1016/0006-291x(86)91224-6. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Marks C. B., Luce J. M., Montgomery A. B., Turner J., Metz C. A., Murray J. F. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am Rev Respir Dis. 1990 Jan;141(1):94–97. doi: 10.1164/ajrccm/141.1.94. [DOI] [PubMed] [Google Scholar]
- Molloy R. G., Mannick J. A., Rodrick M. L. Cytokines, sepsis and immunomodulation. Br J Surg. 1993 Mar;80(3):289–297. doi: 10.1002/bjs.1800800308. [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T. M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P. S., Krönke M., Mak T. W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Sekut L., Menius J. A., Jr, Brackeen M. F., Connolly K. M. Evaluation of the significance of elevated levels of systemic and localized tumor necrosis factor in different animal models of inflammation. J Lab Clin Med. 1994 Dec;124(6):813–820. [PubMed] [Google Scholar]
- Semmler J., Wachtel H., Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int J Immunopharmacol. 1993 Apr;15(3):409–413. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Spengler R. N., Chensue S. W., Giacherio D. A., Blenk N., Kunkel S. L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol. 1994 Mar 15;152(6):3024–3031. [PubMed] [Google Scholar]
- Sprung C. L., Caralis P. V., Marcial E. H., Pierce M., Gelbard M. A., Long W. M., Duncan R. C., Tendler M. D., Karpf M. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984 Nov 1;311(18):1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Ward P. A., Spengler R. N., Lynch J. P., 3rd, Larrick J., Kunkel S. L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- Tannière-Zeller M., Rochette L., Bralet J. PAF-acether-induced mortality in mice: protection by benzodiazepines. Drugs Exp Clin Res. 1989;15(11-12):553–558. [PubMed] [Google Scholar]
- Waage A., Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988 Feb;63(2):299–302. [PMC free article] [PubMed] [Google Scholar]


