Lyme disease (LD) is a multisystem and multistage infection caused by three species of tick-borne spirochetes in the Borrelia burgdorferi sensu lato genogroup. These include B. burgdorferi sensu stricto (North America and Western Europe), Borrelia afzelii (Western Europe, Central Europe, and Russia), and Borrelia garinii (Europe, Russia, and northern Asia). LD has become the most common vector-borne disease in North America and Europe (5). In 1999, over 16,000 cases of human LD were reported in the United States by the Centers for Disease Control and Prevention (CDC), representing an overall incidence of 6.0 per 100,000 persons (9). Many cases go unreported in areas of endimicity; conversely, LD is probably overreported in some geographic areas where the disease is not known to be endemic.
Like other spirochetal infections, the signs and symptoms of LD occur in stages and involve a variety of tissues and organs, including the skin, joints, heart, and nervous system. Early infection (stage 1) consists of primary erythema migrans (EM), an annular skin rash that begins days to weeks after a tick bite. Hematogenous dissemination of spirochetes over subsequent days to weeks (stage 2) can result in multiple skin lesions (secondary EM), as well as meningitis, radiculoneuritis, atrioventricular block, myocarditis, and oligoarticular arthritis. Persistent infection (stage 3) occurs months to years after the initial exposure and can be associated with acrodermatitis chronica atrophicans, varying degrees of encephalopathy and encephalomyelitis, and persistent arthritis. Of note, clinical manifestations of LD among patients in North America seem to differ somewhat from those residing in Europe and Asia. For example, acrodermatitis and severe encephalomyelitis due to LD are more common in Europe and Asia but are infrequent among patients in North America. These discrepancies can be explained, at least in part, by the different genospecies of Borrelia responsible for LD in various geographic areas and possibly by genetic differences among the affected populations (30). Recent reviews provide a comprehensive description of the clinical and epidemiologic aspects of LD (17, 21, 26).
Since the initial description of Lyme arthritis 25 years ago, there have been tremendous gains in knowledge of the pathogenesis, epidemiology, diagnosis, and treatment of LD (29). The CDC has developed a case definition of LD for surveillance purposes that includes either physician-diagnosed EM along with solitary lesions with diameters of at least 5 cm or at least one late joint, neurologic, or cardiac manifestation along with laboratory confirmation (9). This definition is not intended to be 100% sensitive or specific for clinical diagnosis but is useful as a starting point for the development of a differential diagnosis and highlights the central role of laboratory testing, especially for extracutaneous LD.
Laboratory tests have improved considerably over the above-mentioned time period, and clinicians now have available a wide, though somewhat bewildering, array of options for the direct detection of organisms in tissues, serologic detection of immune responses, and molecular detection of specific nucleic acid sequences and antigens. All of the various testing methodologies have their inherent advantages and limitations. It is extremely important to recognize that LD is a clinical diagnosis; any laboratory test used to supplement that evaluation should be ordered and interpreted in the context of careful investigation of the patient's history and physical examination, i.e., after thoughtful assessment of the probability that a patient actually has a borrelial infection. This article reviews the various possibilities for LD testing and emphasizes some practicalities associated with the use of these tests given the present understanding of individual test performance.
CULTURE ISOLATION AND DIRECT DETECTION OF ORGANISMS IN TISSUE
Culture isolation of B. burgdorferi sensu lato from clinical specimens remains the “gold standard” for diagnosis and is most commonly attempted with skin biopsy or cutaneous lavage specimens from EM lesions and blood from patients with early-disseminated disease. Positive culture rates of nearly 90% for secondary EM lesions, 50% for primary EM lesions, and 48% for large-volume (≥9-ml) blood or plasma specimens from patients with early LD have been reported (18, 20, 25, 31). Isolation of B. burgdorferi sensu lato from other sites, such as cerebrospinal fluid (CSF) and synovial fluid, is uncommon; the low recovery rate probably reflects the small number of viable organisms present in those anatomic locations.
Culture of B. burgdorferi sensu lato involves incubating a specimen in Barbour-Stoenner-Kelly medium (BSK) (or modifications of BSK) and detecting the presence of characteristic spirochetes by dark-field microscopy or by fluorescent microscopy with acridine orange or a specific fluorescent antibody (FA). Some microbiologists consider Borrelia culture to be too expensive and tedious to be practical for many clinical laboratories. In reality, isolation of B. burgdorferi sensu lato is no more difficult and is probably easier than recovering and identifying other fastidious microorganisms such as Legionella or Mycobacteria spp. from clinical specimens. BSK is commercially available, and the only specialized equipment required is a fluorescent microscope or a light microscope equipped with a dark-field condenser.
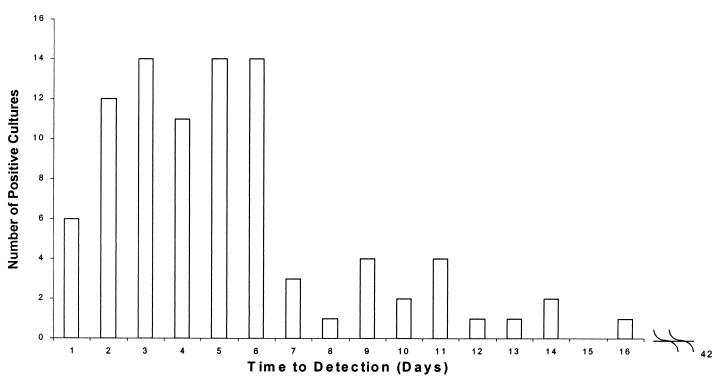
Time to detection of a positive specimen can be within a clinically relevant time frame, provided cultures are examined for the presence of spirochetes at frequent intervals, especially during the first 2 weeks of incubation. Of 90 specimens that were submitted to Marshfield Laboratories from 1991 to 1997 and that turned out to be culture postive, 74 (82%) were identified as positive for Borrelia within the first week of incubation and 32 (35%) were identified within the first 3 days, with the longest time to detection of a positive culture being 16 days (Fig. 1).
FIG. 1.
Time to detection of positive B. burgdorferi culture for 90 specimens submitted to Marshfield Laboratories from 1991 to 1997.
With current methods, Borrelia cultures of skin are most appropriate for untreated patients having lesions suspected to be primary EM, particularly those that appear atypical. The lesions of secondary EM are usually so characteristic that the diagnosis can be made on clinical grounds and the expense of culture is not justified. Culture isolation should also be seriously considered for EM-like lesions associated with tick exposure occurring in a geographical area where an enzootic cycle of B. burgdorferi sensu lato has not been established with certainty. Large-volume plasma cultures are a reasonable consideration in patients without EM who are thought to be at moderate or high risk for early-disseminated LD but who have not yet seroconverted. In most instances, it is not practical to attempt isolation of B. burgdorferi sensu lato from CSF or synovial fluid on a routine basis because immunoserologic or molecular-based tests offer higher sensitivity.
The histopathology of LD is not specific and is most useful for excluding other diseases. Biopsy specimens from of EM lesions show edema, mucin deposition, and a perivascular infiltrate of macrophages, lymphocytes, and occasional plasma cells within the superficial and deep dermis (17). Acute cellulitis due to secondary bacterial infection and allergic reaction to the tick bite can obscure these features and complicate interpretation.
Some investigators have used silver stains (Warthin-Starry, modified Dieterle, or modified Steiner stain), FAs, or immunoperoxidase to localize the loosely coiled organisms within skin and other tissues. These preparations are difficult to interpret because elastic fibers and other tissue filaments can resemble spirochetes. The use of these methodologies should be limited to research and selected clinical situations where experienced observers are available to interpret the results.
IMMUNOLOGIC TESTING
B. burgdorferi sensu stricto is a complex bacterium with numerous immunogenic lipids, proteins, lipoproteins, and carbohydrate antigens on its surface and outer membrane, as well as within the cytoplasm. These antigens offer a wide array of potential targets for immunoserologic testing. Among the most important antigens are the outer surface proteins OspA to -G, the 41-kDa flagellin protein, and a number of heat shock proteins. Some antigens, such as OspA, are lipoproteins expressed by B. burgdorferi sensu lato when the organism resides in the unfed tick vector but are downregulated after a blood meal and upon entry into a human or other mammalian host. The 23-kDa OspC lipoprotein is a highly immunogenic antigen which demonstrates considerable variation, a situation analogous to the variable membrane proteins expressed by other Borrelia species. Although the three genospecies of B. burgdorferi sensu lato express generally similar antigens, significant differences do occur; these differences complicate development of a single immunoserologic assay that is optimal for laboratory testing for LD that has resulted from infection with any one of the genospecies (5, 21, 26).
The immune response to B. burgdorferi sensu lato infection begins with the appearance of specific immunoglobulin M (IgM) antibodies, usually within the first several weeks after initial exposure. The IgM response may persist for many months or years despite effective antimicrobial therapy. Thus, the presence of specific IgM antibodies cannot be used as the sole criterion to diagnose a recent infection. Most patients will have detectable IgG antibodies after 1 month of active infection. Like that of IgM, the IgG response can persist for years after LD symptoms have resolved, and there is no role for the routine use of serologic testing to monitor response to therapy. Both IgG and IgM responses can be greatly diminished or absent in patients receiving antimicrobial therapy early in the course of disease.
The earliest immunoserologic tests for LD were indirect FA (IFA) assays, enzyme-linked immunosorbent assays (ELISAs), and immunodot assays using whole-cell preparations of B. burgdorferi sensu stricto, especially strain B-31. All of these methods are suitable for detecting specific IgM and/or IgG antibodies, but many laboratories find ELISA more convenient for testing large numbers of specimens. In contrast to kits used for human immunodeficiency virus testing, there is little standardization among the numerous commercial kits marketed for LD diagnosis in the United States and Europe (6). When results from different laboratories for well-characterized proficiency samples are compared, significant differences in the sensitivities and specificities of ELISA and IFA have been observed (4).
The numerous antigens present in whole-cell assays can result in cross-reaction with antibodies to other microorganisms or tissue components. Many diseases have been reported to cause significant cross-reactivity in IgM and/or IgG assays. Among such diseases are autoimmune disorders, Epstein-Barr virus infection, bacterial endocarditis, syphilis, other spirochetal infections, and Helicobacter pylori infection.
Some immunoserologic tests that utilize whole-cell preparations of B. burgdorferi sensu lato are modified in order to improve sensitivity and specificity. Modifications can include an adsorption step to block cross-reacting antibodies; enrichment of the antigen source with flagellin, p39, or other B. burgdorferi sensu lato-specific antigens; fractionation of the B. burgdorferi sensu lato antigen source; or the use of an antibody-capture step in an ELISA.
Despite the improvements made in ELISAs and IFAs, they still have the drawback of lacking sensitivity for early disease. Therefore, for patients who have been exposed to ticks in areas of endimicity and who present with characteristic EM, laboratory testing to support the clinical diagnosis of LD is of little value. The decision of whether or not to perform laboratory testing for patients who present without EM but with other objective clinical indications requires an assessment of the probability that the illness is actually LD (6, 32).
The American College of Physicians (ACP) recommends serologic testing for patients with objective clinical signs that have a pretest probability of LD in the range of 0.20 to 0.80 (3). Patients with vague subjective complaints such as headache, fatigue, and myalgia are considered to have a low pretest probability of LD (≤0.20). A positive ELISA result in this setting very likely represents a false-positive result and can lead to misdiagnosis as well as unnecessary and inappropriate use of antimicrobial therapy. Because LD incidence rates and vector abundance vary widely between different geographic areas, it can be difficult for physicians to have sufficient information to allow accurate assessment of pretest probability of LD for individual patients. Exaggerated perceptions of risk by patients and health care providers can result in significant amounts of unnecessary testing and associated expense (5).
Immunoblotting allows detection of antibodies to individual antigens of B. burgdorferi sensu lato and is more specific than ELISA or IFA. Antigens can be derived from whole-cell preparations of B. burgdorferi sensu lato or from expressed proteins taken from recombinant DNA. Both IgM and IgG immunoblotting kits are available, but IgM immunoblotting is less specific than IgG immunoblotting, and patients with symptoms lasting longer than 4 weeks should have only IgG antibody testing done (11).
For the United States, the CDC recommends that all serum specimens for LD diagnosis be evaluated in a two-step process. The first step employs a sensitive serologic test, such as ELISA or IFA. Specimens found to be negative are not tested further. All specimens with positive or equivocal results are tested by immunoblotting, using standardized criteria for interpretation. When immunoblotting is used in the first 4 weeks after the onset of disease, both IgM and IgG procedures should be performed. Most LD patients will seroconvert within this 4-week period. In the event that a patient suspected to have early LD has a negative serology, evidence of infection is best obtained by testing of paired acute- and convalescent-phase samples. Since specific IgG should be present in nearly all untreated patients after 1 month of infection, a positive IgM test result alone cannot be considered to support the diagnosis and may represent a false-positive result. An IgM immunoblot result is considered positive if any 2 of 3 bands (the 23-, 39-, and 41-kDa bands, with the 23-kDa band representing OspC) are present. An IgG immunoblot result is considered positive if any 5 of 10 bands (the 18-, 23-, 28-, 30-, 39-, 41-, 45-, 58-, 66-, and 93-kDa bands, with the 23-kDa band representing OspC) are present (10).
In interpretation of immunoblotting results, the evolution of the immune response in LD should be taken into consideration (1). For example, IgG immunoblotting with an acute-phase serum sample that reveals no bands might be followed several weeks later by IgG immunoblotting with a convalescent-phase serum sample that shows four of the five diagnostic bands. Based on CDC criteria, both tests would be considered negative. However, the results are strongly suggestive of impending seroconversion and, in the proper clinical context, can be supportive of a clinical diagnosis of LD.
In Europe and Asia, the development of a uniform approach for the immunoserologic evaluation of LD is complicated by the presence of organisms from the three genospecies of B. burgdorferi sensu lato genogroup and by significant antigenic variation within each genospecies. Efforts are under way to standardize immunoblotting methodologies and interpretive criteria, but to date there is no consensus. It is possible that for best performance, immunoserologic assays will need to be developed for defined geographic areas based on the specific species and strains of B. burgdorferi sensu lato genogroup organisms that are endemic (14, 24).
Other immunologic assays have been developed to aid in the clinical diagnosis of LD, but overall they have not been as thoroughly evaluated as ELISA, IFA assay, and immunoblot assay. The measurement of T-lymphocyte recognition of B. burgdorferi sensu lato antigens is a relatively complicated assay that has not gained widespread use in clinical practice; reports of the specificity and sensitivity of the assay are variable (21). Detection of circulating immune complexes (IC) has been advocated as an approach to find specific antibodies during very early stages of LD, when excess B. burgdorferi sensu lato antigens bind to IgM antibodies, making them undetectable by standard techniques. Since IC formation requires ongoing B. burgdorferi sensu lato antigen availability, IC-based assays might be useful in distinguishing positive serology associated with active infection from residual antibodies from a prior infection (7). Borreliacidal antibodies are produced early in the course of LD and can be detected by a flow cytometry-based assay available through several reference laboratories. The reported sensitivity is 72% for patients with early LD. Antimicrobial agents present in the serum sample can interfere with the test and must be removed prior to analysis (8).
The vast majority of patients with extracutaneous LD will have detectable serum antibodies. However, occasionally seronegative patients present with a history of tick exposure and rheumatologic or nervous system findings compatible with LD. In some of these cases localized intrathecal or synovial production of specific antibodies has been detected by measuring the ratio of the CSF or joint fluid titer to the serum titer after the samples have been diluted to the same total IgG concentration. A ratio of >1.0 is considered evidence of localized infection. It should be noted that the Food and Drug Administration has cleared over 60 immunoserologic assays for LD for blood, plasma, or serum specimens; to date, none of them are approved for use with CSF or joint fluid (6).
MOLECULAR TESTING
Many clinical laboratories have turned to molecular assays in an attempt to increase sensitivity and specificity and decrease the turnaround time for laboratory testing of LD. The majority of the assays utilize PCR to amplify specific B. burgdorferi sensu lato nucleic acid sequences from tissue biopsy specimens or samples of blood, CSF, joint fluid, or urine. Both single-stage and nested PCR assays have been developed, and detection methods vary from gel electrophoresis and Southern hybridization to real-time PCR with quantitation of product. Both plasmid and chromosomal targets have been used, and each has its advantages. Targets carried on plasmids, such as ospA, ospC, and vlsE, are present in multiple copies within each bacterium, and assays with these targets have greater sensitivity than those employing single-copy chromosomal targets such as fla, recA, rpoB, 16S and 23S ribosomal DNA (rDNA), and the rDNA intergenic spacers. It has been found that in some cases of Lyme arthritis, PCR with joint fluid reveals imbalance between ospA and chromosomal targets such as 16S rDNA. The mechanism accounting for plasmid-carried targets being overabundant compared to chromosomal targets is not known with certainty but could represent nonviable membrane blebs of ospA-carrying plasmids persisting within joint fluid or synovial tissue (23).
PCR can be used to confirm EM lesions before the appearance of serum antibodies and without the delay associated with culture isolation (25). A recent meta-analysis of PCR studies for EM lesions revealed an overall sensitivity of 68% (range, 59 to 84%) and a specificity of 100% (12). Although effective antimicrobial therapy essentially eliminates the ability to isolate B. burgdorferi sensu lato organisms in culture from EM lesions within a few days, the effect on PCR results is less profound because DNA from the organisms can persist in tissues for weeks. For confirmation of early-disseminated disease, PCR has been applied to blood or plasma. Results depend on the stage of illness, with 40% of patients with secondary EM and 9.5% of patients with primary EM yielding positive results (25).
The diagnosis of Lyme arthritis is made based on patient history, physical examination, and, for the majority of patients, a positive immunoserologic test. Patients with persistent symptoms after antimicrobial therapy can be difficult to evaluate because serology does not distinguish between active and inactive infection. PCR has been used successfully to identify B. burgdorferi sensu lato nucleic acids in a high percentage of synovial fluids from patients with untreated Lyme arthritis (22). A meta-analysis of similar studies revealed an overall sensitivity of 73% and specificity of at least 99% (12). Untreated patients have been found to have residual B. burgdorferi sensu lato ospA DNA in the synovial fluid, while patients with persistent arthritis after antimicrobial therapy are unlikely to have detectable plasmid or chromosomal targets. In the latter case, the PCR results support an immune response-mediated etiology for persistent arthritis rather than an active infection requiring additional antimicrobial therapy (28).
Patients with peripheral or central nervous system involvement with LD usually present with symptoms sufficiently long after receiving a tick bite that the result of immunoserologic testing is positive and no additional laboratory confirmation is necessary (21, 26). For the few patients that are seronegative despite having a significant likelihood of infection or for those patients having a particularly confusing array of neurologic symptoms, additional testing is desirable. Because the yield of CSF culture is low, molecular detection by PCR would seem to be an attractive alternative. However, results to date have been highly variable; the overall sensitivity is only in the range of 20% (12). Therefore, a negative result does not rule out the diagnosis of LD.
A controversial area for molecular testing is the detection of specific B. burgdorferi sensu lato antigens or nucleic acids in urine. A Lyme antigen test for urine has been evaluated and was not found to have sufficient reproducibility to be useful in clinical practice (15). In addition, a number of PCR assays have been applied to urine specimens, with sensitivities varying from 13 to 100% (12). Positive PCR results for urine have not always correlated with clinical disease, making it difficult to recommend a rational strategy for their use in clinical diagnosis.
SPECIAL CONSIDERATIONS
Coinfection with other tick-borne pathogens.
Ixodes ticks have a broad host range, therefore increasing the chance of acquiring multiple pathogens from reservoir hosts. This suggests that patients with one documented tick-transmitted disease may be at increased risk for infection with other tick-transmitted microorganisms. In Wisconsin, a study of 96 patients with a primary diagnosis of LD demonstrated immunoserologic evidence of coinfection with Babesia microti and/or Ehrlichia phagocytophila in 9.4% (19). In addition to ehrlichiosis and babesiosis, coinfection with tick-borne encephalitis virus is a possible consideration for LD patients in Europe. Infection with multiple agents could explain, at least in part, the variable manifestations and clinical responses reported in some patients with LD and may have confounded our understanding of the true clinical spectrum of the disease. As a practical consideration, laboratory testing for coinfection should be considered in some clinical situations to ensure that appropriate antimicrobial therapy is prescribed.
Laboratory testing in vaccinated individuals.
In 1998 the Food and Drug Administration approved a recombinant OspA vaccine for use in patients (age range, 15 to 70 years) at risk of acquiring LD (27). Since the efficacy of the vaccine is reported to be in the range of 76% after three doses, there will be instances where vaccinated individuals acquire natural infection with B. burgdorferi sensu lato. ELISAs and IFA assays utilizing OspA-producing strains of B. burgdorferi sensu lato will not discriminate between vaccinated and naturally infected individuals. Therefore, the CDC two-step algorithm is not applicable to vaccinated patients and immunoblotting must be relied on for serologic confirmation of infection. In addition to increasing costs, the issue is complicated further by reports that some vaccinated patients produce antibodies that bind to various borrelial proteins, making interpretation of immunoblots more difficult (2). This will continue to be problematic until ELISAs and other first-line assays based on non-OspA-producing strains or recombinant borrelial proteins are in widespread use and physicians become aware of the specific limitations of the testing methods provided by their laboratories (13, 15, 16).
Tick assays.
Clinical laboratories are sometimes requested to identify ticks removed from the skin or clothing of patients and to test them for the presence or absence of B. burgdorferi sensu lato. PCR assays, IFA assays, and culture have been used to detect B. burgdorferi sensu lato in ticks, but the value of this information in the clinical setting is extremely limited. A positive result has a low positive predictive value for the subsequent development of LD in the exposed patient, and a negative result does not preclude transmission of infection from the submitted tick or other ticks that the patient was not aware of. Further, testing of ticks for B. burgdorferi sensu lato provides no information about coinfection with other tick-borne pathogens.
SUMMARY
Laboratory testing in support of the clinical diagnosis of LD is indicated for some patients assessed to have a moderate to high risk of acquiring the disease based on exposure history and objective clinical signs and symptoms. Typical primary or secondary EM can be treated empirically and does not require laboratory confirmation. Culture isolation of B. burgdorferi sensu lato from skin can be accomplished in a clinically relevant time frame and should be considered for atypical EM and for EM-like lesions in patients from areas of nonendimicity. Supplemental immunoserologic testing is indicated for symptomatic patients at significant risk for extracutaneous LD. In the United States, the CDC two-step protocol (ELISA or IFA assay with confirmation of positive or equivocal results by immunoblotting) improves specificity and provides sufficient information to allow rational patient management decisions in the majority of cases. Detection of natural B. burgdorferi sensu lato infection in OspA-vaccinated patients is problematic with many of the presently available ELISA and IFA tests.
Molecular-based tests hold promise for improving diagnostic accuracy and decreasing turnaround time for results. However, assessment of the probability of borrelial infection is equally important for PCR-based tests as it is for immunoserologic assays. The ability to detect specific B. burgdorferi sensu lato DNA in the synovial fluid of treated patients with Lyme arthritis can help to distinguish active infection from persistent symptoms due to an immunologic mechanism.
Acknowledgments
I thank S. Shukla, P. Mitchell, E. Belongia, and G. Eldred for critical comments and Sharon Brock for help in preparing the manuscript.
This work was supported in part by grants from the Marshfield Medical Research Foundation.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld, M. E., J. Roberge, C. A. Carbonaro, J. Nowakowski, R. B. Nadelman, and G. P. Wormser. 1999. Effects of OspA vaccination on Lyme disease serologic testing. J. Clin. Microbiol. 37:3718-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Physicians. 1997. Guidelines for laboratory evaluation in the diagnosis of Lyme disease. Ann. Int. Med. 127:1106-1108. [DOI] [PubMed] [Google Scholar]
- 4.Bakken, L. L., S. M. Callister, P. J. Wand, and R. F. Schell. 1997. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists Proficiency Testing Program. J. Clin. Microbiol. 35:537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G. 1998. Fall and rise of Lyme disease and other Ixodes tick-borne infections in North America and Europe. Br. Med. Bull. 54:647-658. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S. L., S. L. Hansen, and J. J. Langone. 1999. Role of serology in the diagnosis of Lyme disease. JAMA 282:62-66. [DOI] [PubMed] [Google Scholar]
- 7.Brunner, M., and L. H. Sigal. 2001. Use of serum immune complexes in a new test that accurately confirms early Lyme disease and active infection with Borrelia burgdorferi. J. Clin. Microbiol. 39:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2001. Lyme Disease—United States 1999. Morb. Mort. Wkly. Rep. 50:181-185. [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morb. Mort. Wkly. Rep. 44:590.. [PubMed] [Google Scholar]
- 11.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 12.Dumler, J. S. 2001. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol. Diagn. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 13.Gomes-Solecki, M. J., J. J. Dunn, B. J. Luft, J. Castillo, D. E. Dykhuizen, X. Yang, J. D. Glass, and R. J. Dattwyler. 2000. Recombinant chimeric Borrelia proteins for diagnosis of Lyme disease. J. Clin. Microbiol. 38:2530-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauser, U., G. Lehnert, R. Lobentanzer, and B. Wilske. 1997. Interpretation criteria for standardized western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 35:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klempner, M. S., C. H. Schmid, L. Hu, A. C. Steere, G. Johnson, B. McCloud, R. Noring, and A. Weinstein. 2001. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am. J. Med. 110:217-219. [DOI] [PubMed] [Google Scholar]
- 16.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melski, J. W. 2000. Lyme borreliosis. Semin. Cutan. Med. Surg. 19:10-18. [DOI] [PubMed] [Google Scholar]
- 18.Melski, J. W., K. D. Reed, P.D. Mitchell, and G. D. Barth. 1993. Primary and secondary erythema migrans in central Wisconsin. Arch. Dermatol. 129:709-716. [PubMed] [Google Scholar]
- 19.Mitchell, P. D., K. D. Reed, and J. M. Hofkes. 1996. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J. Clin. Microbiol. 34:724-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell, P. D., K. D. Reed, M. F. Vandermause, and J. W. Melski. 1993. Isolation of Borrelia burgdorferi from skin biopsy specimens of patients with erythema migrans. Am. J. Clin. Pathol. 99:104-107. [DOI] [PubMed] [Google Scholar]
- 21.Nadelman, R. B., and G. P. Wormser. 1998. Lyme borreliosis. Lancet 352:557-565. [DOI] [PubMed] [Google Scholar]
- 22.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 23.Persing, D. H., B. J. Rutledge, P. N. Rys, D. S. Podzorski, P. D. Mitchell, K. D. Reed, B. Liu, E. Fikrig, and S. E. Malawista. 1994. Target imbalance: disparity of Borrelia burgdorferi genetic material in synovial fluid from Lyme arthritis patients. J. Infect. Dis. 169:668-672. [DOI] [PubMed] [Google Scholar]
- 24.Robertson, J., E. Guy, N. Andrews, B. Wilske, P. Anda, M. Granstrom, U. Hauser, Y. Moosmann, V. Sambri, J. Schellekens, G. Stanek, and J. Gray. 2000. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 38:2097-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz, I., G. P. Wormser, J. J. Schwartz, D. Cooper, P. Weissensee, A. Gazumyan, E. Zimmermann, N. S. Goldberg, S. Bittker, G. L. Campbell, and C. S. Pavia. 1992. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J. Clin. Microbiol. 30:3082-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 27.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, and D. S. Krause. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme disease vaccine study group. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 28.Steere, A. C., D. Gross, A. L. Meyer, and B. T. Huber. 2001. Autoimmune mechanisms in antibiotic treatment-resistant Lyme arthritis. J. Autoimmun. 16:263-268. [DOI] [PubMed] [Google Scholar]
- 29.Steere, A. C., S. E. Malawista, D. R. Snydman, R. E. Shope, W. A. Andiman, M. R. Ross, and F. M. Steele. 1977. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 20:7-17. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaaard, A. C. P. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 31.Wormser, G. P., S. Bittker, D. Cooper, J. Nowakowski, R. B. Nadelman, and C. Pavia. 2001. Yield of large-volume blood cultures in patients with early Lyme disease. J. Infect. Dis. 184:1070-1072. [DOI] [PubMed] [Google Scholar]
- 32.Wormser, G. P., M. E. Aguero-Rosenfeld, and R. B. Nadelman. 1999. Lyme disease serology problems and opportunities. JAMA 282:79-80. [DOI] [PubMed] [Google Scholar]