Abstract
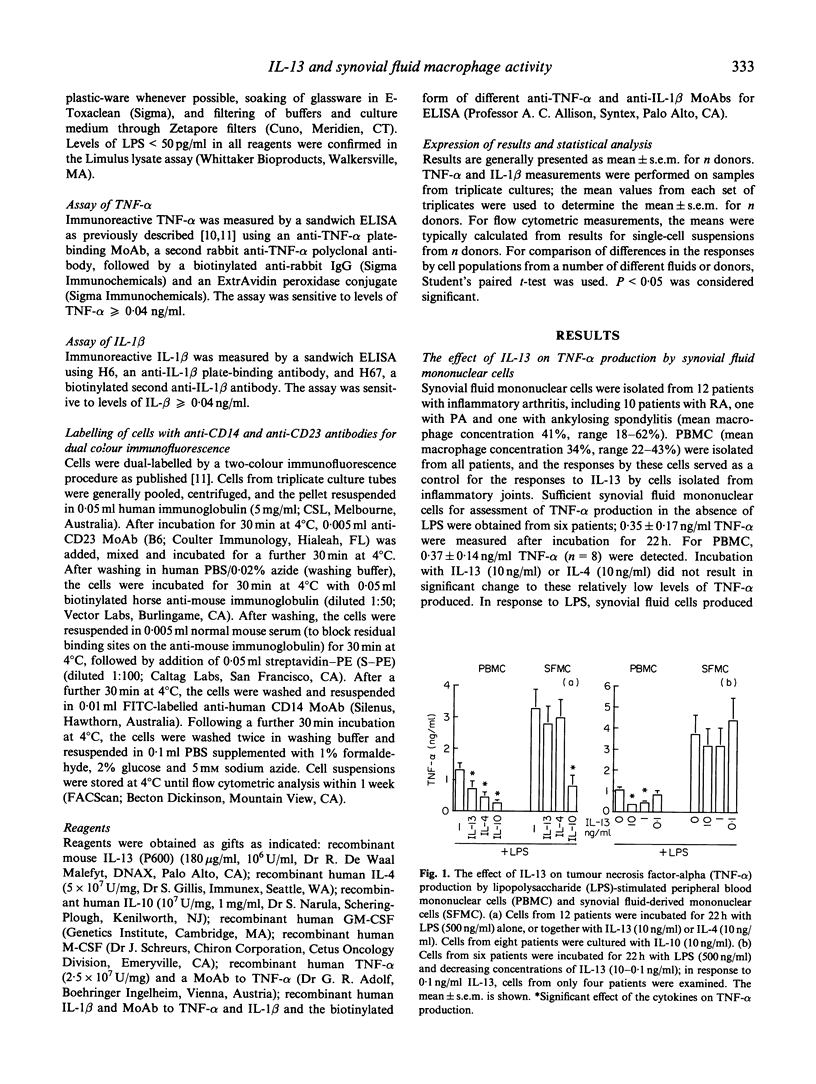
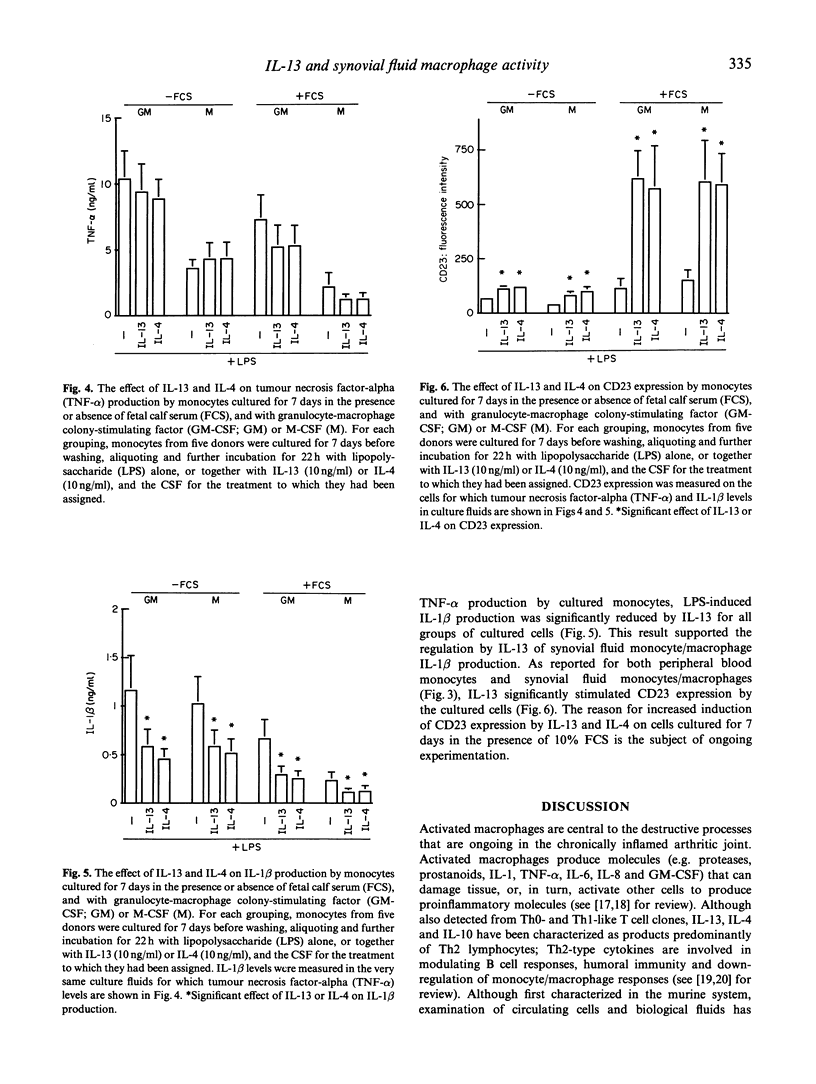
Activated macrophages are central to the destructive processes of chronic inflammatory arthritis. In this study, it was hypothesized that IL-13, a product predominantly of 'Th2-type' lymphocytes, may be used therapeutically to down-regulate monocyte/macrophage activities at sites of chronic inflammation. Synovial fluid mononuclear cells were isolated from 12 patients with chronic inflammatory arthritis. Peripheral blood mononuclear cells (PBMC) were isolated at the same time as synovial fluid cells from all 12 patients. IL-13 significantly inhibited lipopolysaccharide (LPS)-induced tumour necrosis factor-alpha (TNF-alpha) production by mononuclear cells from peripheral blood, but not synovial fluid. In contrast, IL-13 inhibited LPS-induced IL-1 beta production by all cells, and as a positive response to IL-13, CD23 expression was increased on both cell populations. Blood monocytes cultured for 7 days with granulocyte-macrophage colony-stimulating factor (GM-CSF) or M-CSF responded to IL-13 in a manner similar to that detected for synovial fluid-derived cells, with suppression of LPS-induced IL-1 beta, but not TNF-alpha, production. In all experiments, the responses to IL-13 were very similar to those detected to IL-4, but differed from those measured with IL-10. Thus, the responses to IL-13 by synovial fluid cells and cultured monocytes are not equal to those of blood monocytes. The similar responses to IL-4 and IL-13 support claims of a common element for signalling from the IL-4 and IL-13 receptors. Furthermore, the activity of a common receptor chain may be altered by monocyte activation and differentiation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Brugger W., Scheibenbogen C., Kreutz M., Leser H. G., Rehm A., Löhr G. W. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990 Jun;47(6):490–497. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Aversa G., Punnonen J., Cocks B. G., de Waal Malefyt R., Vega F., Jr, Zurawski S. M., Zurawski G., de Vries J. E. An interleukin 4 (IL-4) mutant protein inhibits both IL-4 or IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: support for a common component shared by IL-4 and IL-13 receptors. J Exp Med. 1993 Dec 1;178(6):2213–2218. doi: 10.1084/jem.178.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Hakim F. T., Venzon D. J., Blatt S., Hendrix C. W., Wynn T. A., Shearer G. M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993 Mar;91(3):759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks B. G., de Waal Malefyt R., Galizzi J. P., de Vries J. E., Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993 Jun;5(6):657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- Duff G. W. Cytokines and anti-cytokines. Br J Rheumatol. 1993 Mar;32 (Suppl 1):15–20. [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Vogel S. N. Differential production of IFN-alpha/beta by CSF-1- and GM-CSF-derived macrophages. J Leukoc Biol. 1990 Jul;48(1):43–49. doi: 10.1002/jlb.48.1.43. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Ahern M. J., Jones C. A., Jones K. L., Smith M. D., Finlay-Jones J. J. Synovial fluid macrophages and blood monocytes differ in their response to IL-4. J Immunol. 1993 Sep 15;151(6):3370–3380. [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- McKenzie A. N., Culpepper J. A., de Waal Malefyt R., Brière F., Punnonen J., Aversa G., Sato A., Dang W., Cocks B. G., Menon S. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie A. N., Li X., Largaespada D. A., Sato A., Kaneda A., Zurawski S. M., Doyle E. L., Milatovich A., Francke U., Copeland N. G. Structural comparison and chromosomal localization of the human and mouse IL-13 genes. J Immunol. 1993 Jun 15;150(12):5436–5444. [PubMed] [Google Scholar]
- Minty A., Chalon P., Derocq J. M., Dumont X., Guillemot J. C., Kaghad M., Labit C., Leplatois P., Liauzun P., Miloux B. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993 Mar 18;362(6417):248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Nutman T. B. Type 2 cytokines and negative immune regulation in human infections. Curr Opin Immunol. 1993 Aug;5(4):511–517. doi: 10.1016/0952-7915(93)90031-m. [DOI] [PubMed] [Google Scholar]
- Panayi G. S. The immunopathogenesis of rheumatoid arthritis. Br J Rheumatol. 1993 Mar;32 (Suppl 1):4–14. [PubMed] [Google Scholar]
- Peleman R., Wu J., Fargeas C., Delespesse G. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J Exp Med. 1989 Nov 1;170(5):1751–1756. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J., Aversa G., Cocks B. G., McKenzie A. N., Menon S., Zurawski G., de Waal Malefyt R., de Vries J. E. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993 Aug;5(4):524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993 Dec 17;262(5141):1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Rutherford M. S., Witsell A., Schook L. B. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993 May;53(5):602–618. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Modlin R. L., Ohmen J. D., Moy R. L. Local expression of antiinflammatory cytokines in cancer. J Clin Invest. 1993 Mar;91(3):1005–1010. doi: 10.1172/JCI116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Young D. A., Lowe L. D., Clark S. C. Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J Immunol. 1990 Jul 15;145(2):607–615. [PubMed] [Google Scholar]
- Zurawski G., de Vries J. E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994 Jan;15(1):19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Zurawski S. M., Vega F., Jr, Huyghe B., Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993 Jul;12(7):2663–2670. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C. G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J. E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993 Dec 1;151(11):6370–6381. [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]


