Abstract
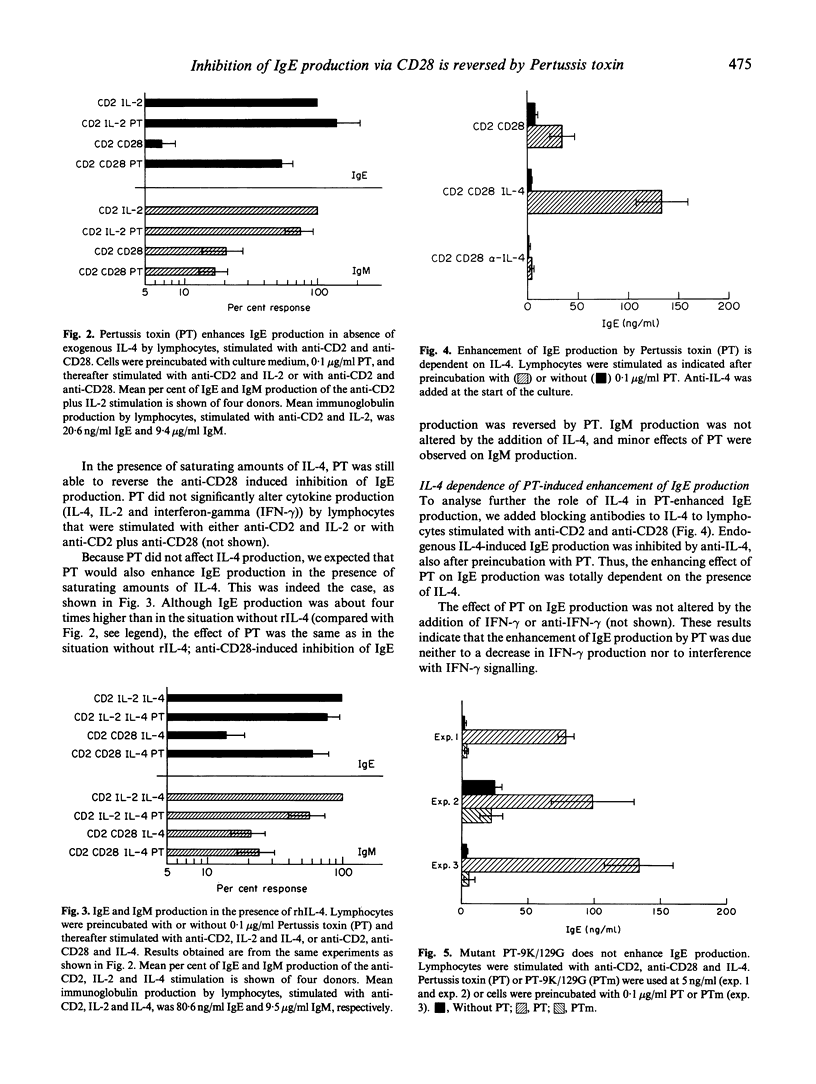
In lymphocyte cultures, IgE production was achieved by stimulating T cells with anti-CD2 and IL-2. Here we show that anti-CD28, in the presence or absence of IL-2, reduces this IgE production approximately 10-fold. This inhibition of IgE production was almost completely reversed by Pertussis toxin (PT). PT had no effect on IgE production when the cells were stimulated in the absence of anti-CD28. No major effects of PT were found on IgM production. PT had no effect on purified B cells, stimulated with IL-4 and anti-CD40. In the presence of saturating amounts of rIL-4 similar results were obtained, albeit the absolute amounts of IgE produced were higher in all situations. Furthermore, PT-induced IgE production was still dependent on IL-4, as was evident from experiments in which anti-IL-4 was added to the culture. The IgE enhancing effect was dependent on the adenosine diphosphate (ADP)-ribosyltransferase activity of PT, because a mutant molecule lacking this activity was not able to restore anti-CD28-induced inhibition of IgE synthesis. Thus, we show that co-stimulation with anti-CD28 causes an inhibition of T cell-dependent IgE production by B cells, which inhibition can be specifically overcome by PT. An analysis of the molecular pathways underlying this phenomenon may contribute to our understanding of the regulation of IgE synthesis in (patho)physiological conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Gilmore W., Weiner L. P. The effects of pertussis toxin and cholera toxin on mitogen-induced interleukin-2 production: evidence for G protein involvement in signal transduction. Cell Immunol. 1988 May;113(2):235–250. doi: 10.1016/0008-8749(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein regulation of receptor signalling. Immunol Today. 1988 Oct;9(10):315–320. doi: 10.1016/0167-5699(88)91325-4. [DOI] [PubMed] [Google Scholar]
- Hedenskog S., Björkstén B., Blennow M., Granström G., Granström M. Immunoglobulin E response to pertussis toxin in whooping cough and after immunization with a whole-cell and an acellular pertussis vaccine. Int Arch Allergy Appl Immunol. 1989;89(2-3):156–161. doi: 10.1159/000234939. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kolb J. P., Genot E., Petit-Koskas E., Paul-Eugene N., Dugas B. Effect of bacterial toxins on human B cell activation. I. Mitogenic activity of pertussis toxin. Eur J Immunol. 1990 May;20(5):969–976. doi: 10.1002/eji.1830200504. [DOI] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto J., Breittmayer J. P., Grenier-Brossette N., Fehlmann M., Cousin J. L. Pertussis toxin-sensitive G-proteins are not involved in activation of T-lymphocytes. Cell Signal. 1991;3(1):25–33. doi: 10.1016/0898-6568(91)90004-e. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Jin Y. J., Stebbins C. C., Lopez P. A., Alcover A., Reinherz E. L. Characterization of functional GTP binding proteins in Jurkat T cell mutants lacking either CD3-Ti or CD2 surface receptors. Cell Immunol. 1990 Jul;128(2):578–588. doi: 10.1016/0008-8749(90)90050-2. [DOI] [PubMed] [Google Scholar]
- Mu H. H., Sewell W. A. Enhancement of interleukin-4 production by pertussis toxin. Infect Immun. 1993 Jul;61(7):2834–2840. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Peacock M. G. Action of pertussigen (pertussis toxin) on serum IgE and on Fc epsilon receptors on lymphocytes. Cell Immunol. 1990 May;127(2):327–336. doi: 10.1016/0008-8749(90)90136-f. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Nencioni L., Pizza M., Bugnoli M., De Magistris T., Di Tommaso A., Giovannoni F., Manetti R., Marsili I., Matteucci G., Nucci D. Characterization of genetically inactivated pertussis toxin mutants: candidates for a new vaccine against whooping cough. Infect Immun. 1990 May;58(5):1308–1315. doi: 10.1128/iai.58.5.1308-1315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Van der Straeten M., Platteau B., Bazin H. The non-specific enhancement of allergy. I. In vivo effects of Bordetella pertussis vaccine on IgE synthesis. Allergy. 1983 May;38(4):239–246. doi: 10.1111/j.1398-9995.1983.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Pizza M., Covacci A., Bartoloni A., Perugini M., Nencioni L., De Magistris M. T., Villa L., Nucci D., Manetti R., Bugnoli M. Mutants of pertussis toxin suitable for vaccine development. Science. 1989 Oct 27;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- Punnonen J., Aversa G., Cocks B. G., McKenzie A. N., Menon S., Zurawski G., de Waal Malefyt R., de Vries J. E. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso A., Smilovich D., Capra M. C., Baldissarro I., Yan G., Bargellesi A., Cosulich M. E. CD69 in resting and activated T lymphocytes. Its association with a GTP binding protein and biochemical requirements for its expression. J Immunol. 1991 Jun 15;146(12):4105–4114. [PubMed] [Google Scholar]
- Rogers T. S., Corey S. J., Rosoff P. M. Identification of a 43-kilodalton human T lymphocyte membrane protein as a receptor for pertussis toxin. J Immunol. 1990 Jul 15;145(2):678–683. [PubMed] [Google Scholar]
- Sauerwein R. W., Mudde G. C., Vooys W. C., van der Meer W. G., de Gast G. C., Aarden L. A. Induction of IgM production by pokeweed mitogen and interleukin-2: different responses by human peripheral blood cells and tonsil cells. Clin Immunol Immunopathol. 1986 Jun;39(3):431–441. doi: 10.1016/0090-1229(86)90171-6. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990 Feb;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint F. G., Kaliński P., Wierenga E. A., Bos J. D., Kapsenberg M. L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993 Jun 15;150(12):5321–5329. [PubMed] [Google Scholar]
- Stewart S. J., Prpic V., Johns J. A., Powers F. S., Graber S. E., Forbes J. T., Exton J. H. Bacterial toxins affect early events of T lymphocyte activation. J Clin Invest. 1989 Jan;83(1):234–242. doi: 10.1172/JCI113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Huston G., Tonkonogy S., Weinberg A. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991 Nov 1;147(9):2991–3000. [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Torre D., Sampietro C., Fiori G. P., Ferraro G., Chelazzi G., Issi M. Total serum IgE levels in children with pertussis. Lancet. 1989 May 20;1(8647):1148–1148. doi: 10.1016/s0140-6736(89)92436-7. [DOI] [PubMed] [Google Scholar]
- Van der Pouw-Kraan T., Van Kooten C., Rensink I., Aarden L. Interleukin (IL)-4 production by human T cells: differential regulation of IL-4 vs. IL-2 production. Eur J Immunol. 1992 May;22(5):1237–1241. doi: 10.1002/eji.1830220519. [DOI] [PubMed] [Google Scholar]
- Van der Pouw-Kraan T., Van Kooten C., Van Oers R., Aarden L. A. Human transferrin allows efficient IgE production by anti-CD3-stimulated human lymphocytes at low cell densities. Eur J Immunol. 1991 Feb;21(2):385–390. doi: 10.1002/eji.1830210220. [DOI] [PubMed] [Google Scholar]
- Vercelli D., Geha R. S. Regulation of IgE synthesis in humans: a tale of two signals. J Allergy Clin Immunol. 1991 Sep;88(3 Pt 1):285–295. doi: 10.1016/0091-6749(91)90087-5. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Geerts M., Aarden L. A. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem. 1991 Aug 5;266(22):14179–14182. [PubMed] [Google Scholar]
- Witvliet M. H., Vogel M. L., Wiertz E. J., Poolman J. T. Interaction of pertussis toxin with human T lymphocytes. Infect Immun. 1992 Dec;60(12):5085–5090. doi: 10.1128/iai.60.12.5085-5090.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer M., Roos D. Metabolic comparison between basophils and other leukocytes from human blood. J Immunol. 1986 May 1;136(9):3447–3454. [PubMed] [Google Scholar]
- de Vries J. E., Gauchat J. F., Aversa G. G., Punnonen J., Gascan H., Yssel H. Regulation of IgE synthesis by cytokines. Curr Opin Immunol. 1991 Dec;3(6):851–858. doi: 10.1016/s0952-7915(05)80003-2. [DOI] [PubMed] [Google Scholar]
- van Kooten C., Rensink I., Pascual-Salcedo D., van Oers R., Aarden L. Monokine production by human T cells; IL-1 alpha production restricted to memory T cells. J Immunol. 1991 Apr 15;146(8):2654–2658. [PubMed] [Google Scholar]


