Abstract
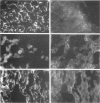
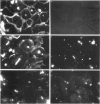
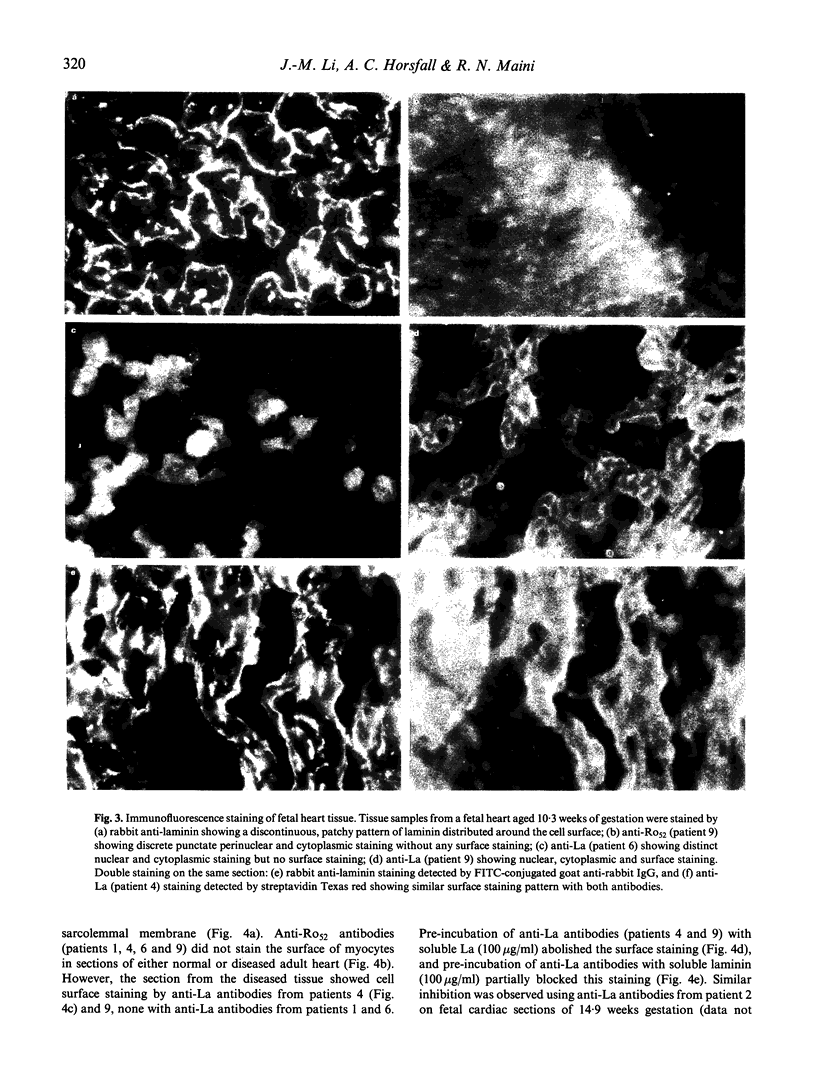
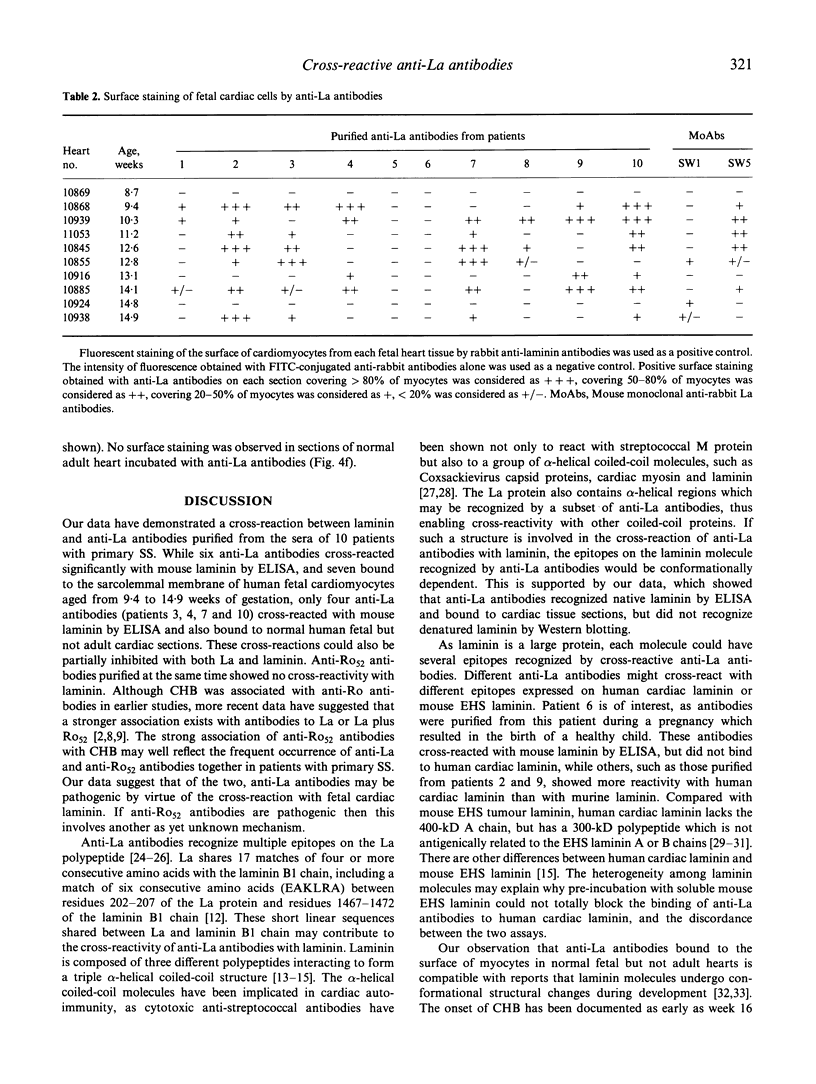
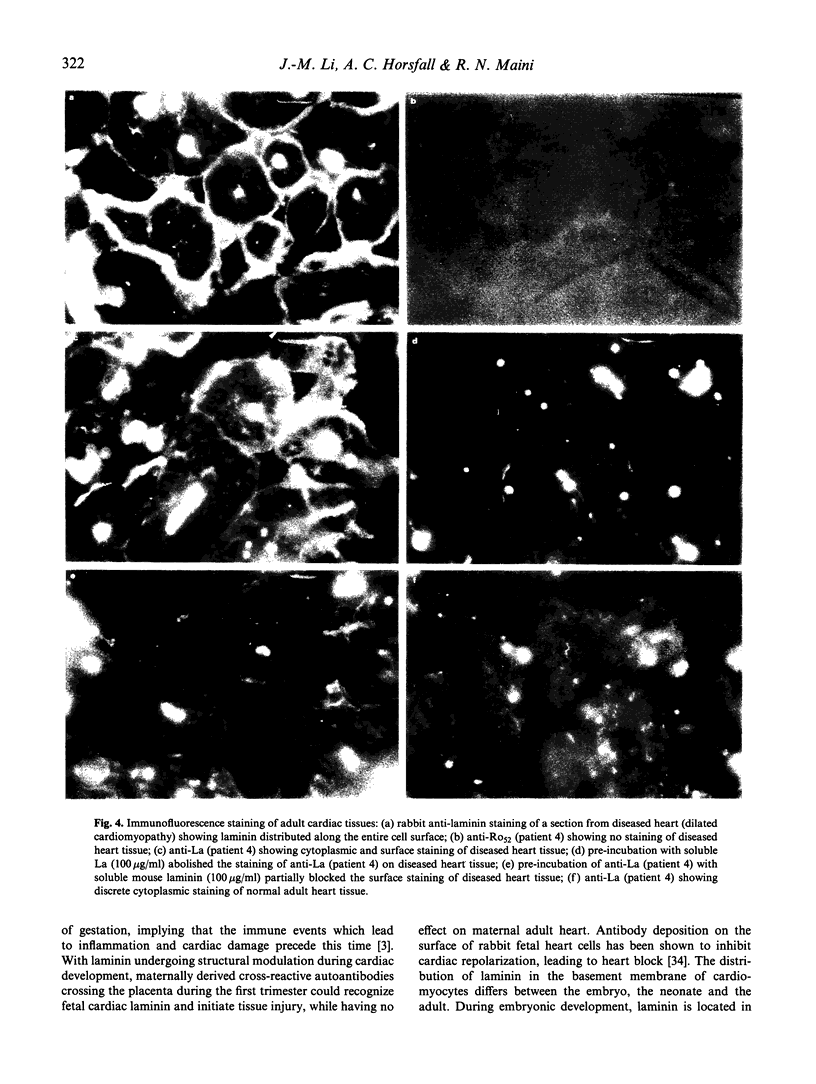
Cross-reactions between maternally derived autoantibodies and fetal cardiac antigens have been postulated to play a role in the pathogenesis of congenital heart block (CHB). We have explored the cross-reactivity of autoantibodies to the small ribonuclear autoantigens, La/SS-B and Ro/SS-A, with laminin, the major component of cardiac sarcolemmal membrane using affinity-purified antibodies from patients with Sjögren's syndrome (SS). Anti-La antibodies purified from eight of 10 patients cross-reacted significantly with mouse laminin by ELISA. In contrast, purified antibodies to Ro52 from the same 10 patients showed little or no binding to laminin. Laminin inhibited up to 70% binding of anti-La antibodies to La antigen, and La inhibited up to 65% binding of anti-La antibodies to laminin. The cross-reaction was further examined on cryosections of 10 human fetal hearts aged from 8.7 to 14.9 weeks of gestation, two normal adult hearts, and one pathological adult heart with a diagnosis of dilated cardiomyopathy. Anti-Ro52 antibodies did not bind to the surface of cardiac cells. However, anti-La antibodies from seven of 10 patients tested bound to the surface of fetal myocytes from hearts aged 9.4 to 14.9 weeks of gestation, and also to the myocytes from the pathological adult heart but not to normal adult hearts. Preincubation with La antigen abolished the binding of anti-La antibodies to the surface of adult heart myocytes with dilated cardiomyopathy, and pre-incubation with mouse laminin could partially block this binding. These results suggest that molecular mimicry between laminin and La, but not Ro52, may act as a target for specific maternal autoantibodies, and contribute to the pathogenesis of CHB at a critical stage during fetal cardiac development.
Full text
PDF

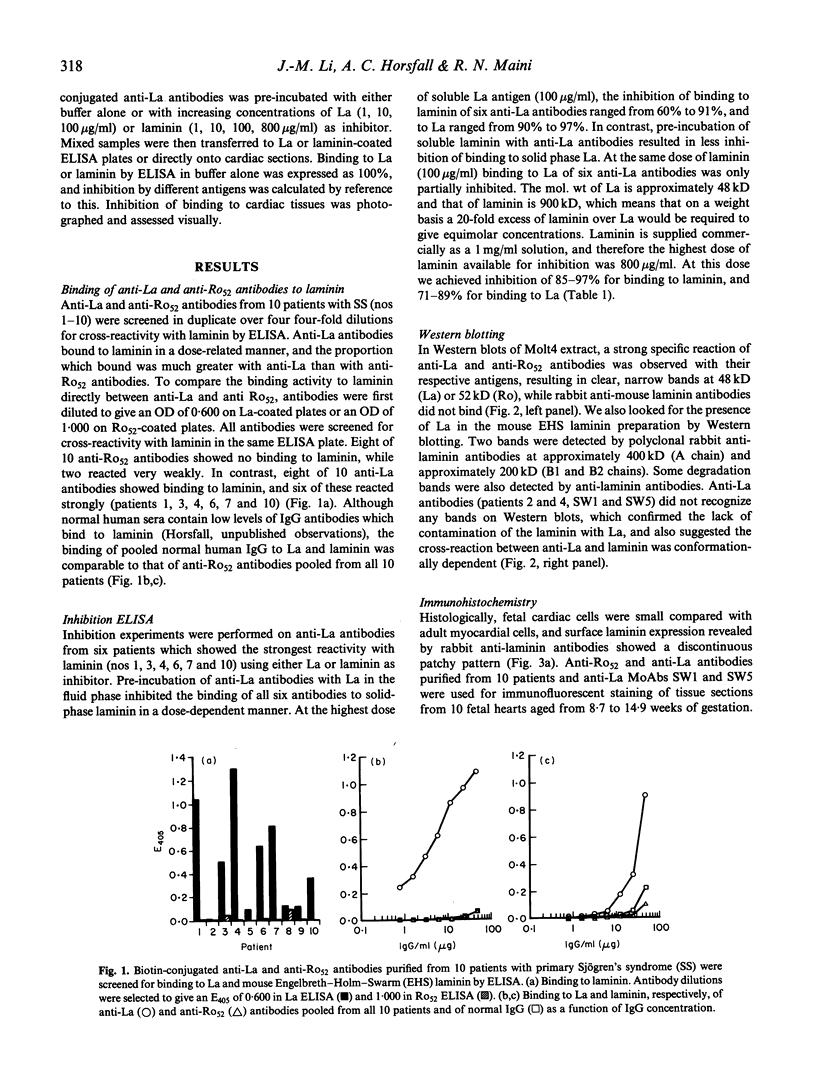
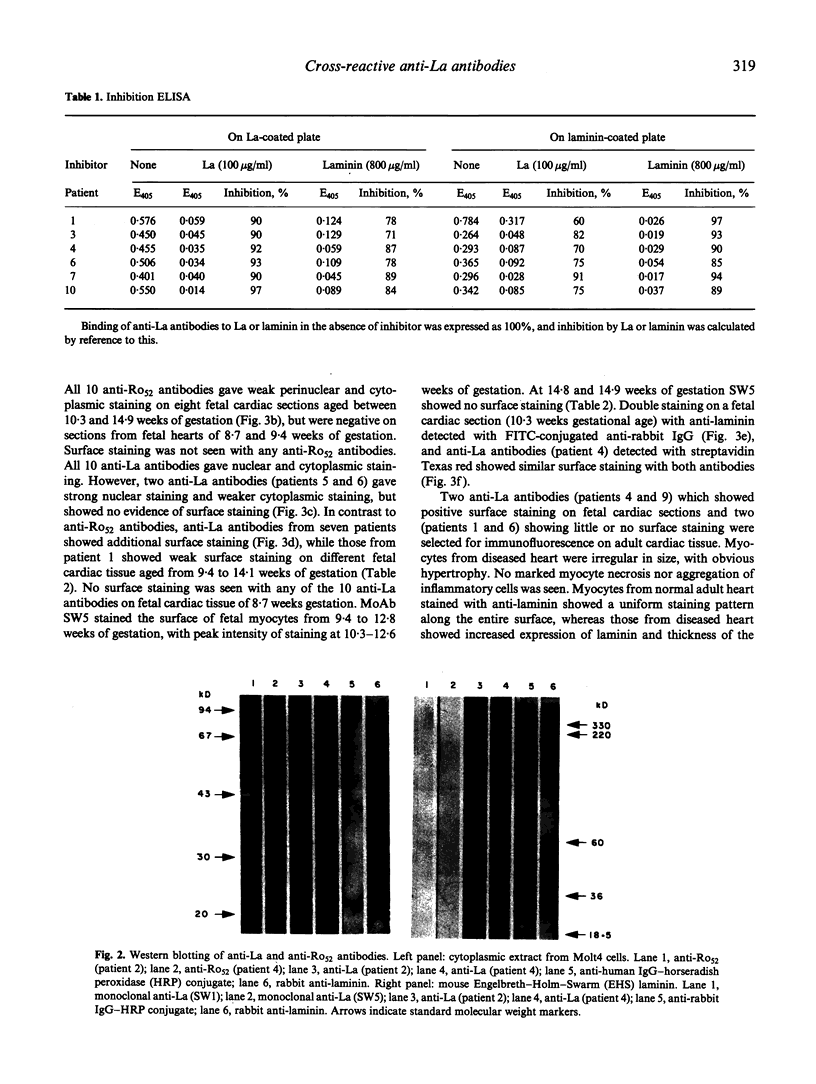


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Buyon J. P., Lane J., Lafond-Walker A., Provost T. T., Guarnieri T. Anti-SS-A/Ro SS-B/La antibodies bind to neonatal rabbit cardiac cells and preferentially inhibit in vitro cardiac repolarization. J Autoimmun. 1989 Aug;2(4):463–469. doi: 10.1016/0896-8411(89)90176-5. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Pfeifer K., Schröder H. C., Müller W. E. Characterization of the autoantigen La as a nucleic acid-dependent ATPase/dATPase with melting properties. Cell. 1990 Jan 12;60(1):85–93. doi: 10.1016/0092-8674(90)90718-t. [DOI] [PubMed] [Google Scholar]
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990 Feb 1;4(2):148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Bini P., Chu J. L., Okolo C., Elkon K. Analysis of autoantibodies to recombinant La (SS-B) peptides in systemic lupus erythematosus and primary Sjogren's syndrome. J Clin Invest. 1990 Feb;85(2):325–333. doi: 10.1172/JCI114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon J. P., Ben-Chetrit E., Karp S., Roubey R. A., Pompeo L., Reeves W. H., Tan E. M., Winchester R. Acquired congenital heart block. Pattern of maternal antibody response to biochemically defined antigens of the SSA/Ro-SSB/La system in neonatal lupus. J Clin Invest. 1989 Aug;84(2):627–634. doi: 10.1172/JCI114208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon J. P., Slade S. G., Chan E. K., Tan E. M., Winchester R. Effective separation of the 52 kDa SSA/Ro polypeptide from the 48 kDa SSB/La polypeptide by altering conditions of polyacrylamide gel electrophoresis. J Immunol Methods. 1990 May 25;129(2):207–210. doi: 10.1016/0022-1759(90)90440-7. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., MacQueen H. A. Subunits of laminin are differentially synthesized in mouse eggs and early embryos. Dev Biol. 1983 Apr;96(2):467–471. doi: 10.1016/0012-1606(83)90183-5. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., Antone S. M., Gulizia J. M., McManus B. A., Gauntt C. J. Alpha-helical coiled-coil molecules: a role in autoimmunity against the heart. Clin Immunol Immunopathol. 1993 Aug;68(2):118–123. doi: 10.1006/clin.1993.1106. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Talaber L. R., Harley J. B., Ayoub E. M., Muneer R. S., Chun L. T., Reddy D. V. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J Immunol. 1988 Oct 15;141(8):2760–2766. [PubMed] [Google Scholar]
- Dörner T., Trebeljahr G., Göldner B., Yamamoto K., Apostoloff E., Hiepe F. Detection of autoantibodies to Ro(SS-A), La(SS-B) and U1RNP in different congenital heart rhythm disorders using immunoblot and enzyme immunoassay. J Autoimmun. 1994 Feb;7(1):93–106. doi: 10.1006/jaut.1994.1007. [DOI] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Gaither K. K. Autoantibodies. Rheum Dis Clin North Am. 1988 Apr;14(1):43–56. [PubMed] [Google Scholar]
- Horsfall A. C., Brown C. M., Maini R. N. Purification of human autoantibodies from cross-linked antigen immunosorbents. J Immunol Methods. 1987 Nov 23;104(1-2):43–49. doi: 10.1016/0022-1759(87)90485-6. [DOI] [PubMed] [Google Scholar]
- Horsfall A. C., Rose L. M. Cross-reactive maternal autoantibodies and congenital heart block. J Autoimmun. 1992 Aug;5(4):479–493. doi: 10.1016/0896-8411(92)90007-d. [DOI] [PubMed] [Google Scholar]
- Horsfall A. C., Rose L. M., Maini R. N. Autoantibody synthesis in salivary glands of Sjögren's syndrome patients. J Autoimmun. 1989 Aug;2(4):559–568. doi: 10.1016/0896-8411(89)90189-3. [DOI] [PubMed] [Google Scholar]
- Horsfall A. C., Venables P. J., Taylor P. V., Maini R. N. Ro and La antigens and maternal anti-La idiotype on the surface of myocardial fibres in congenital heart block. J Autoimmun. 1991 Feb;4(1):165–176. doi: 10.1016/0896-8411(91)90015-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNeilage L. J., Umapathysivam K., Macmillan E., Guidolin A., Whittingham S., Gordon T. Definition of a discontinuous immunodominant epitope at the NH2 terminus of the La/SS-B ribonucleoprotein autoantigen. J Clin Invest. 1992 May;89(5):1652–1656. doi: 10.1172/JCI115762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen M., Vuolteenaho R., Boot-Handford R., Kallunki P., Tryggvason K. Primary structure of the human laminin A chain. Limited expression in human tissues. Biochem J. 1991 Jun 1;276(Pt 2):369–379. doi: 10.1042/bj2760369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Saladin K., Engvall E. Structure of laminin variants. The 300-kDa chains of murine and bovine heart laminin are related to the human placenta merosin heavy chain and replace the a chain in some laminin variants. J Biol Chem. 1991 Sep 15;266(26):17545–17551. [PubMed] [Google Scholar]
- Paulsson M., Saladin K. Mouse heart laminin. Purification of the native protein and structural comparison with Engelbreth-Holm-Swarm tumor laminin. J Biol Chem. 1989 Nov 5;264(31):18726–18732. [PubMed] [Google Scholar]
- Price R. L., Nakagawa M., Terracio L., Borg T. K. Ultrastructural localization of laminin on in vivo embryonic, neonatal, and adult rat cardiac myocytes and in early rat embryos raised in whole-embryo culture. J Histochem Cytochem. 1992 Sep;40(9):1373–1381. doi: 10.1177/40.9.1506674. [DOI] [PubMed] [Google Scholar]
- Ramsey-Goldman R., Hom D., Deng J. S., Ziegler G. C., Kahl L. E., Steen V. D., LaPorte R. E., Medsger T. A., Jr Anti-SS-A antibodies and fetal outcome in maternal systemic lupus erythematosus. Arthritis Rheum. 1986 Oct;29(10):1269–1273. doi: 10.1002/art.1780291013. [DOI] [PubMed] [Google Scholar]
- Ross B. A. Congenital complete atrioventricular block. Pediatr Clin North Am. 1990 Feb;37(1):69–78. doi: 10.1016/s0031-3955(16)36832-8. [DOI] [PubMed] [Google Scholar]
- Saetersdal T., Larsen T., Rotevatn S., Dalen H., Scheie P. Fibronectin and laminin in transverse tubules of cardiac myocytes studied by laser confocal microscopy and immunocytochemistry. Histochemistry. 1992 Sep;98(2):73–80. doi: 10.1007/BF00716997. [DOI] [PubMed] [Google Scholar]
- Silverman E., Mamula M., Hardin J. A., Laxer R. Importance of the immune response to the Ro/La particle in the development of congenital heart block and neonatal lupus erythematosus. J Rheumatol. 1991 Jan;18(1):120–124. [PubMed] [Google Scholar]
- Smith P. R., Williams D. G., Venables P. J., Maini R. N. Monoclonal antibodies to the Sjögren's syndrome associated antigen SS-B (La). J Immunol Methods. 1985 Feb 28;77(1):63–76. doi: 10.1016/0022-1759(85)90184-x. [DOI] [PubMed] [Google Scholar]
- Speiser B., Weihrauch D., Riess C. F., Schaper J. The extracellular matrix in human cardiac tissue. Part II: Vimentin, laminin, and fibronectin. Cardioscience. 1992 Mar;3(1):41–49. [PubMed] [Google Scholar]
- St Clair E. W., Burch J. A., Jr, Ward M. M., Keene J. D., Pisetsky D. S. Temporal correlation of antibody responses to different epitopes of the human La autoantigen. J Clin Invest. 1990 Feb;85(2):515–521. doi: 10.1172/JCI114467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. V., Scott J. S., Gerlis L. M., Esscher E., Scott O. Maternal antibodies against fetal cardiac antigens in congenital complete heart block. N Engl J Med. 1986 Sep 11;315(11):667–672. doi: 10.1056/NEJM198609113151103. [DOI] [PubMed] [Google Scholar]
- Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989 Apr 1;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- Venables P. J., Charles P. J., Buchanan R. R., Yi T., Mumford P. A., Schrieber L., Room G. R., Maini R. N. Quantitation and detection of isotypes of anti-SS-B antibodies by ELISA and Farr assays using affinity purified antigens. An approach to the investigation of Sjögren's syndrome and systemic lupus erythematosus. Arthritis Rheum. 1983 Feb;26(2):146–155. doi: 10.1002/art.1780260205. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C., Chung A. E., Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984 Mar;98(3):971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A., Vermeulen J. L., Verbeek F. J., Virágh S., Kálmán F., Lamers W. H., Moorman A. F. Spatial distribution of "tissue-specific" antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat Rec. 1992 Jan;232(1):97–111. doi: 10.1002/ar.1092320111. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Tichy D., Damjanov A., Paulsson M., Damjanov I. Distinct antigenic characteristics of murine parietal yolk sac laminin. Dev Biol. 1987 Jun;121(2):397–407. doi: 10.1016/0012-1606(87)90176-x. [DOI] [PubMed] [Google Scholar]