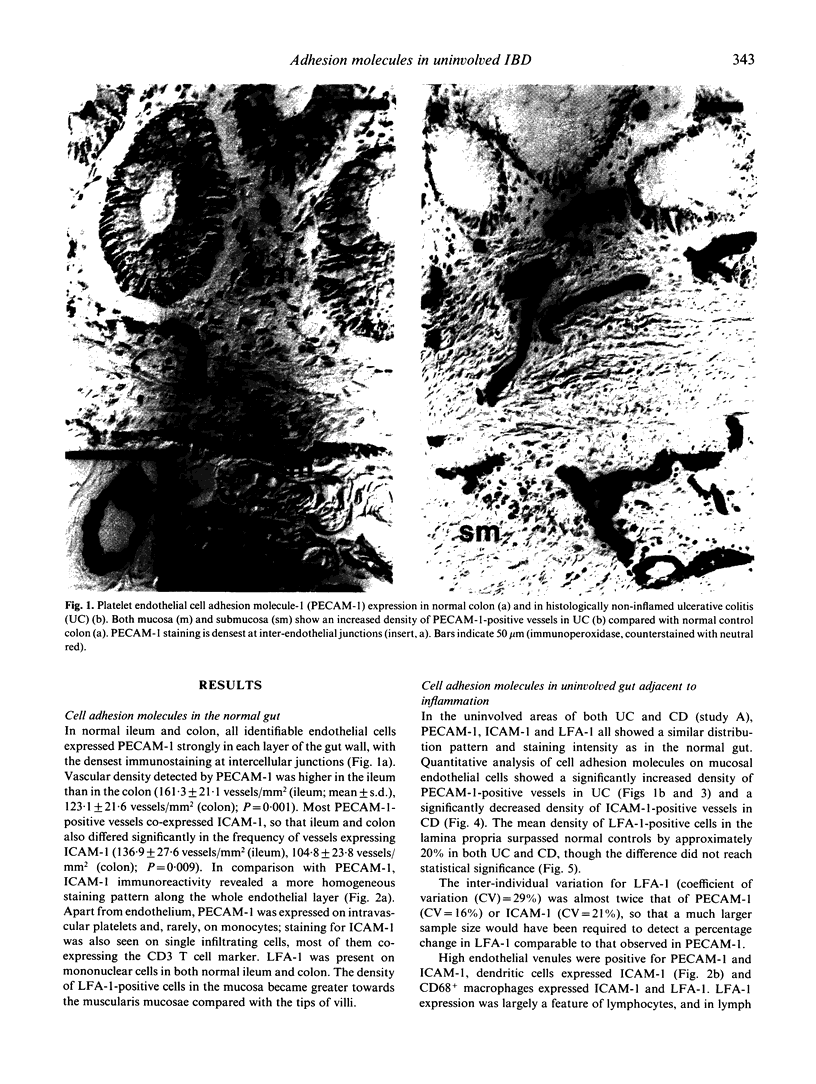
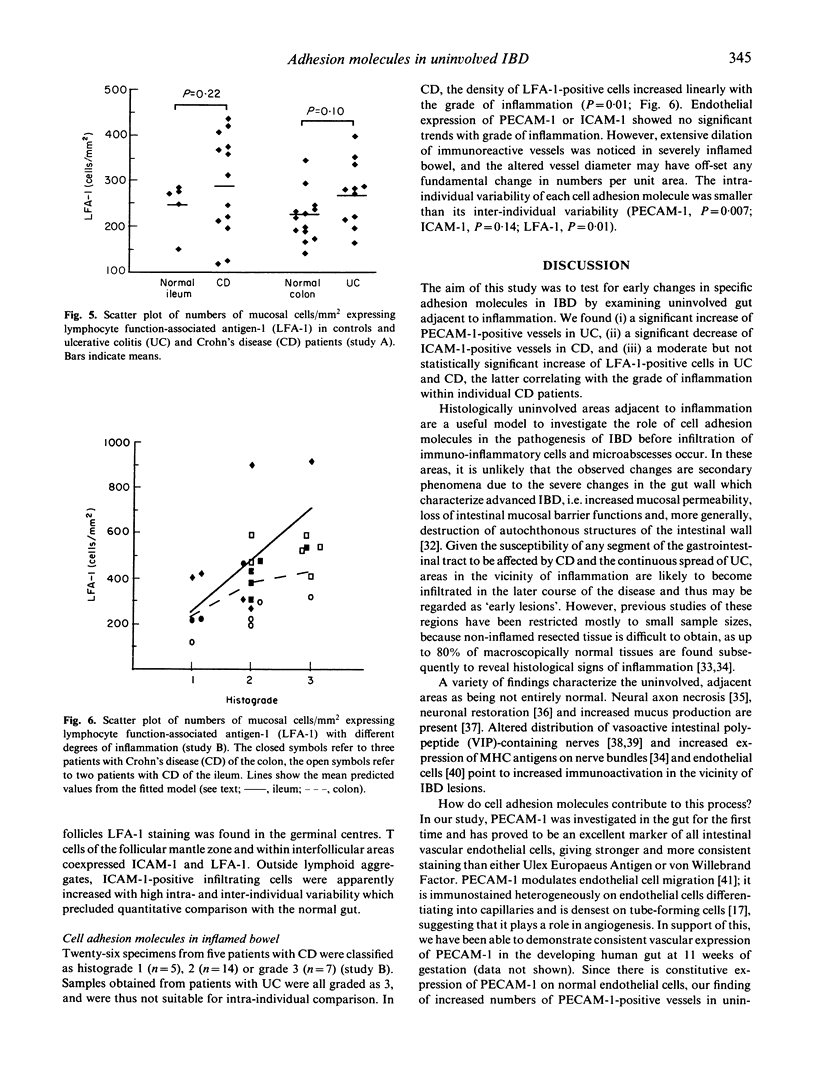
Abstract
Adhesion of circulating cells to vascular endothelium occurs in the early phase of inflammation, and is mediated by specific cell adhesion molecules. Many such adhesion molecules are increased in inflamed regions of ulcerative colitis (UC) and Crohn's disease (CD) but there is limited knowledge of their expression in the uninvolved gut, adjacent to inflammation. We investigated immunohistochemically the expression of platelet endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1) and lymphocyte function-associated antigen-1 (LFA-1) on resected specimens taken at a distance of 2-4 cm from the inflamed area and without histological signs of inflammation. Compared with normal gut, we found (i) a significant increase of PECAM-1-positive vessels in the mucosa of uninvolved UC (149.0 +/- 24.1 vessels/mm2 (mean +/- s.d.); normal colon = 123.1 +/- 21.6; P = 0.004); (ii) a significant decrease of ICAM-1-positive vessels in uninvolved CD (111.9 +/- 22.6 vessels/mm2; normal ileum = 136.9 +/- 27.6; P = 0.04); and (iii) a moderate but statistically insignificant increase of LFA-1-positive cells in the mucosa of uninvolved UC and Crohn's ileitis. This altered expression of cell adhesion molecules may contribute to the early lesion in inflammatory bowel disease and provide new therapeutic opportunities.
Full text
PDF

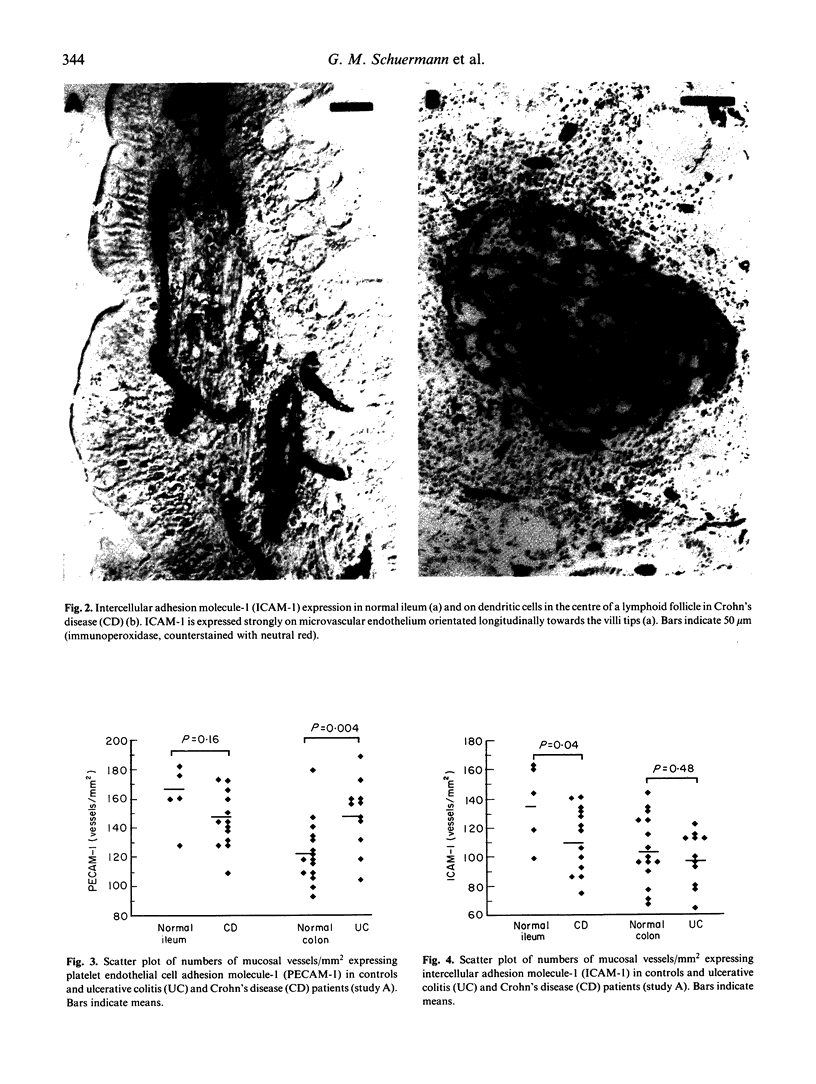


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Oliver P. D., Romer L. H., Buck C. A. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990 Apr;110(4):1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Bryant M. G., Bloom S. R., Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980 Nov;79(5 Pt 1):853–860. [PubMed] [Google Scholar]
- Choy M. Y., Richman P. I., Horton M. A., MacDonald T. T. Expression of the VLA family of integrins in human intestine. J Pathol. 1990 Jan;160(1):35–40. doi: 10.1002/path.1711600109. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A. P., Anthony A., Sim R., Wakefield A. J., Sankey E. A., Hudson M., Allison M. C., Pounder R. E. Mucosal capillary thrombi in rectal biopsies. Histopathology. 1992 Aug;21(2):127–133. doi: 10.1111/j.1365-2559.1992.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Silen W. Differentiation between Crohn's disease and other inflammatory conditions by electron microscopy. Ann Surg. 1985 Jan;201(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- Geboes K., Rutgeerts P., Ectors N., Mebis J., Penninckx F., Vantrappen G., Desmet V. J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology. 1992 Aug;103(2):439–447. doi: 10.1016/0016-5085(92)90832-j. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kaiserlian D., Rigal D., Abello J., Revillard J. P. Expression, function and regulation of the intercellular adhesion molecule-1 (ICAM-1) on human intestinal epithelial cell lines. Eur J Immunol. 1991 Oct;21(10):2415–2421. doi: 10.1002/eji.1830211018. [DOI] [PubMed] [Google Scholar]
- Koizumi M., King N., Lobb R., Benjamin C., Podolsky D. K. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992 Sep;103(3):840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Koretz K., Momburg F., Otto H. F., Möller P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am J Pathol. 1987 Dec;129(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Horton M. A., Choy M. Y., Richman P. I. Increased expression of laminin/collagen receptor (VLA-1) on epithelium of inflamed human intestine. J Clin Pathol. 1990 Apr;43(4):313–315. doi: 10.1136/jcp.43.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malizia G., Calabrese A., Cottone M., Raimondo M., Trejdosiewicz L. K., Smart C. J., Oliva L., Pagliaro L. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):150–159. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- Mazurov A. V., Vinogradov D. V., Kabaeva N. V., Antonova G. N., Romanov Y. A., Vlasik T. N., Antonov A. S., Smirnov V. N. A monoclonal antibody, VM64, reacts with a 130 kDa glycoprotein common to platelets and endothelial cells: heterogeneity in antibody binding to human aortic endothelial cells. Thromb Haemost. 1991 Oct 1;66(4):494–499. [PubMed] [Google Scholar]
- Montefort S., Feather I. H., Wilson S. J., Haskard D. O., Lee T. H., Holgate S. T., Howarth P. H. The expression of leukocyte-endothelial adhesion molecules is increased in perennial allergic rhinitis. Am J Respir Cell Mol Biol. 1992 Oct;7(4):393–398. doi: 10.1165/ajrcmb/7.4.393. [DOI] [PubMed] [Google Scholar]
- Montefort S., Roche W. R., Howarth P. H., Djukanovic R., Gratziou C., Carroll M., Smith L., Britten K. M., Haskard D., Lee T. H. Intercellular adhesion molecule-1 (ICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in the bronchial mucosa of normal and asthmatic subjects. Eur Respir J. 1992 Jul;5(7):815–823. [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Hillery C. A., Albrecht R., Parise L. V., Berndt M. C., Mazurov A. V., Dunlop L. C., Zhang J., Rittenhouse S. E. Activation-dependent changes in human platelet PECAM-1: phosphorylation, cytoskeletal association, and surface membrane redistribution. J Cell Biol. 1992 Oct;119(1):239–246. doi: 10.1083/jcb.119.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Morain C., Bishop A. E., McGregor G. P., Levi A. J., Bloom S. R., Polak J. M., Peters T. J. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984 Jan;25(1):57–61. doi: 10.1136/gut.25.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani H., Nakamura S., Watanabe Y., Fukushima K., Mizoi T., Kimura M., Hiwatashi N., Nagura H. Light and electron microscopic immunolocalization of endothelial leucocyte adhesion molecule-1 in inflammatory bowel disease. Morphological evidence of active synthesis and secretion into vascular lumen. Virchows Arch A Pathol Anat Histopathol. 1992;420(5):403–409. doi: 10.1007/BF01600511. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. Regulation of lymphocyte traffic to mucosa-associated lymphatic tissues. Gastroenterol Clin North Am. 1991 Sep;20(3):495–510. [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Schimmenti L. A., Yan H. C., Madri J. A., Albelda S. M. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J Cell Physiol. 1992 Nov;153(2):417–428. doi: 10.1002/jcp.1041530222. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Walker C., Power C., Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990 Jun 1;171(6):2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart C. J., Calabrese A., Oakes D. J., Howdle P. D., Trejdosiewicz L. K. Expression of the LFA-1 beta 2 integrin (CD11a/CD18) and ICAM-1 (CD54) in normal and coeliac small bowel mucosa. Scand J Immunol. 1991 Sep;34(3):299–305. doi: 10.1111/j.1365-3083.1991.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Rose M. L., Yacoub M. H., Pigott R. Induction of vascular adhesion molecules during rejection of human cardiac allografts. Transplantation. 1992 Sep;54(3):451–457. doi: 10.1097/00007890-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988 Mar;81(3):939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. Inhibition of leukocyte adherence by anti-CD18 monoclonal antibody attenuates reperfusion injury in the rabbit ear. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2643–2646. doi: 10.1073/pnas.87.7.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield A. J., Sawyerr A. M., Dhillon A. P., Pittilo R. M., Rowles P. M., Lewis A. A., Pounder R. E. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989 Nov 4;2(8671):1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]
- Webberley M. J., Hart M. T., Melikian V. Thromboembolism in inflammatory bowel disease: role of platelets. Gut. 1993 Feb;34(2):247–251. doi: 10.1136/gut.34.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Newman W., Shimizu Y., Nutman T. B., Tanaka Y., Horgan K. J., Gopal T. V., Ennis E., O'Sullivan D., Grey H. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991 Oct 1;174(4):901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]