Abstract
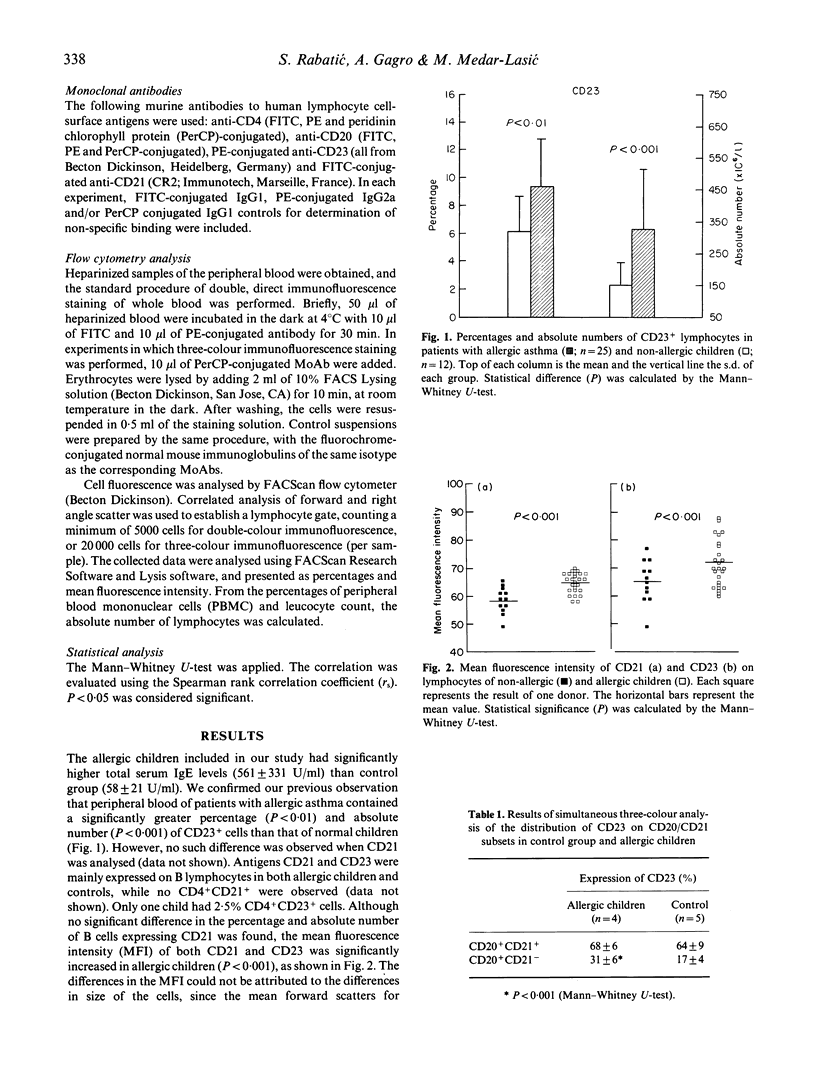
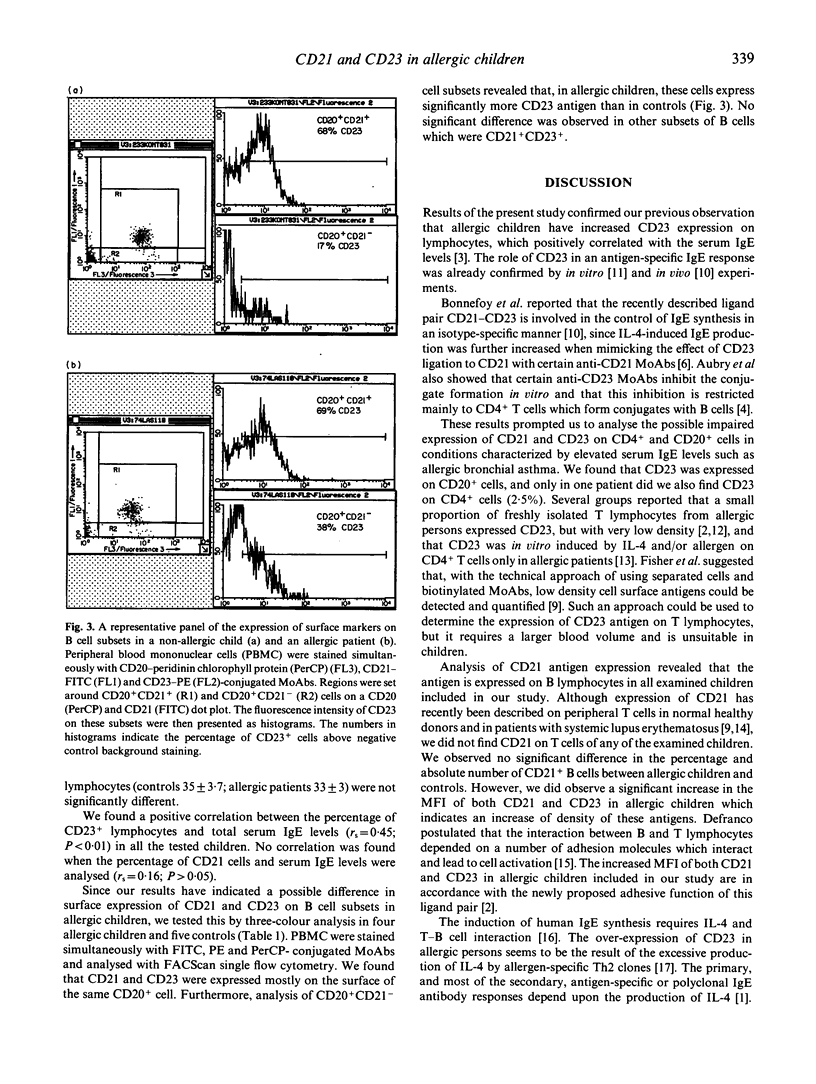
The CD23 antigen, a low affinity receptor for IgE, was recently shown to interact with another ligand, CD21, and the pairing of these molecules is important in T cell-B cell interaction and control of IgE production. Here, we analysed the expression of CD21 and CD23 on CD4+ and CD20+ lymphocytes in 25 allergic children and 12 age-matched non-allergic controls. Both the percentage (P < 0.01) and the absolute number (P < 0.001) of CD23+ cells were increased in allergic children. There was no difference of CD21+ cells. Double positive CD4+ CD23+ cells (2.5%) were only detected in one patient, in others all CD23 being expressed on B cells. The CD21 antigen was expressed only on B cells. Furthermore, allergic children had an increased mean fluorescence intensity of both the CD21 (P < 0.001) and the CD23 (P < 0.001) receptor. To analyse the possible difference in B cell subsets expressing CD21 and CD23 antigens, three-colour fluorescence analysis was performed. In allergic children the subset of CD20+ CD21- cells expressed more CD23 than in controls (P < 0.001). These results may mean an impaired expression and possibly regulation of CD21-CD23 interaction in allergic conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afar B., Merrill J., Clark E. A. Detection of lymphocyte subsets using three-color/single-laser flow cytometry and the fluorescent dye peridinin chlorophyll-alpha protein. J Clin Immunol. 1991 Sep;11(5):254–261. doi: 10.1007/BF00918183. [DOI] [PubMed] [Google Scholar]
- Aubry J. P., Pochon S., Graber P., Jansen K. U., Bonnefoy J. Y. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992 Aug 6;358(6386):505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- Aubry J. P., Shields J. G., Jansen K. U., Bonnefoy J. Y. A multiparameter flow cytometric method to study surface molecules involved in interactions between subpopulations of cells. J Immunol Methods. 1993 Feb 26;159(1-2):161–171. doi: 10.1016/0022-1759(93)90154-y. [DOI] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Pochon S., Aubry J. P., Graber P., Gauchat J. F., Jansen K., Flores-Romo L. A new pair of surface molecules involved in human IgE regulation. Immunol Today. 1993 Jan;14(1):1–2. doi: 10.1016/0167-5699(93)90313-A. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Moore M. D., Nemerow G. R. Immunobiology of CR2, the B lymphocyte receptor for Epstein-Barr virus and the C3d complement fragment. Annu Rev Immunol. 1988;6:85–113. doi: 10.1146/annurev.iy.06.040188.000505. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L. Between B cells and T cells. Nature. 1991 Jun 20;351(6328):603–604. doi: 10.1038/351603a0. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. The CD19-CR2-TAPA-1 complex, CD45 and signaling by the antigen receptor of B lymphocytes. Curr Opin Immunol. 1993 Jun;5(3):341–348. doi: 10.1016/0952-7915(93)90051-s. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Fischer E., Delibrias C., Kazatchkine M. D. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991 Feb 1;146(3):865–869. [PubMed] [Google Scholar]
- Gagro A., Rabatić S., Tresćec A., Dekaris D., Medar-Lasić M. Expression of lymphocytes Fc epsilon RII/CD23 in allergic children undergoing hyposensitization. Int Arch Allergy Immunol. 1993;101(2):203–208. doi: 10.1159/000236520. [DOI] [PubMed] [Google Scholar]
- Gordon J., Flores-Romo L., Cairns J. A., Millsum M. J., Lane P. J., Johnson G. D., MacLennan I. C. CD23: a multi-functional receptor/lymphokine? Immunol Today. 1989 May;10(5):153–157. doi: 10.1016/0167-5699(89)90171-0. [DOI] [PubMed] [Google Scholar]
- Levy E., Ambrus J., Kahl L., Molina H., Tung K., Holers V. M. T lymphocyte expression of complement receptor 2 (CR2/CD21): a role in adhesive cell-cell interactions and dysregulation in a patient with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1992 Nov;90(2):235–244. doi: 10.1111/j.1365-2249.1992.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. G., Sandor M., Nunez R., Mathur A., Hagen M., Waldschmidt T., Van Ness B., Nelms K., Noben N., Ibraghimov A. Lymphocyte Fc receptors: the immunobiology and pathology of CD23. Immunobiology. 1992 Aug;185(2-4):235–267. doi: 10.1016/S0171-2985(11)80644-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Takahashi H., Miike T. Increase of T cells bearing Fc epsilon R-associated antigen in patients with atopic asthma. Ann Allergy. 1987 Apr;58(4):261–264. [PubMed] [Google Scholar]
- McClure J. E. Cellular receptor for Epstein-Barr virus. Prog Med Virol. 1992;39:116–138. [PubMed] [Google Scholar]
- Pochon S., Graber P., Yeager M., Jansen K., Bernard A. R., Aubry J. P., Bonnefoy J. Y. Demonstration of a second ligand for the low affinity receptor for immunoglobulin E (CD23) using recombinant CD23 reconstituted into fluorescent liposomes. J Exp Med. 1992 Aug 1;176(2):389–397. doi: 10.1084/jem.176.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz J. C., Baur X., Mazur G., Rieber E. P. Allergen-directed expression of Fc receptors for IgE (CD23) on human T lymphocytes is modulated by interleukin 4 and interferon-gamma. Eur J Immunol. 1990 Jun;20(6):1259–1264. doi: 10.1002/eji.1830200610. [DOI] [PubMed] [Google Scholar]
- Sherr E., Macy E., Kimata H., Gilly M., Saxon A. Binding the low affinity Fc epsilon R on B cells suppresses ongoing human IgE synthesis. J Immunol. 1989 Jan 15;142(2):481–489. [PubMed] [Google Scholar]
- Tan H. P., Lebeck L. K., Nehlsen-Cannarella S. L. Regulatory role of cytokines in IgE-mediated allergy. J Leukoc Biol. 1992 Jul;52(1):115–118. doi: 10.1002/jlb.52.1.115. [DOI] [PubMed] [Google Scholar]
- Vercelli D., Geha R. S. Regulation of IgE synthesis in humans: a tale of two signals. J Allergy Clin Immunol. 1991 Sep;88(3 Pt 1):285–295. doi: 10.1016/0091-6749(91)90087-5. [DOI] [PubMed] [Google Scholar]
- Wierenga E. A., Snoek M., Jansen H. M., Bos J. D., van Lier R. A., Kapsenberg M. L. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991 Nov 1;147(9):2942–2949. [PubMed] [Google Scholar]


