Abstract
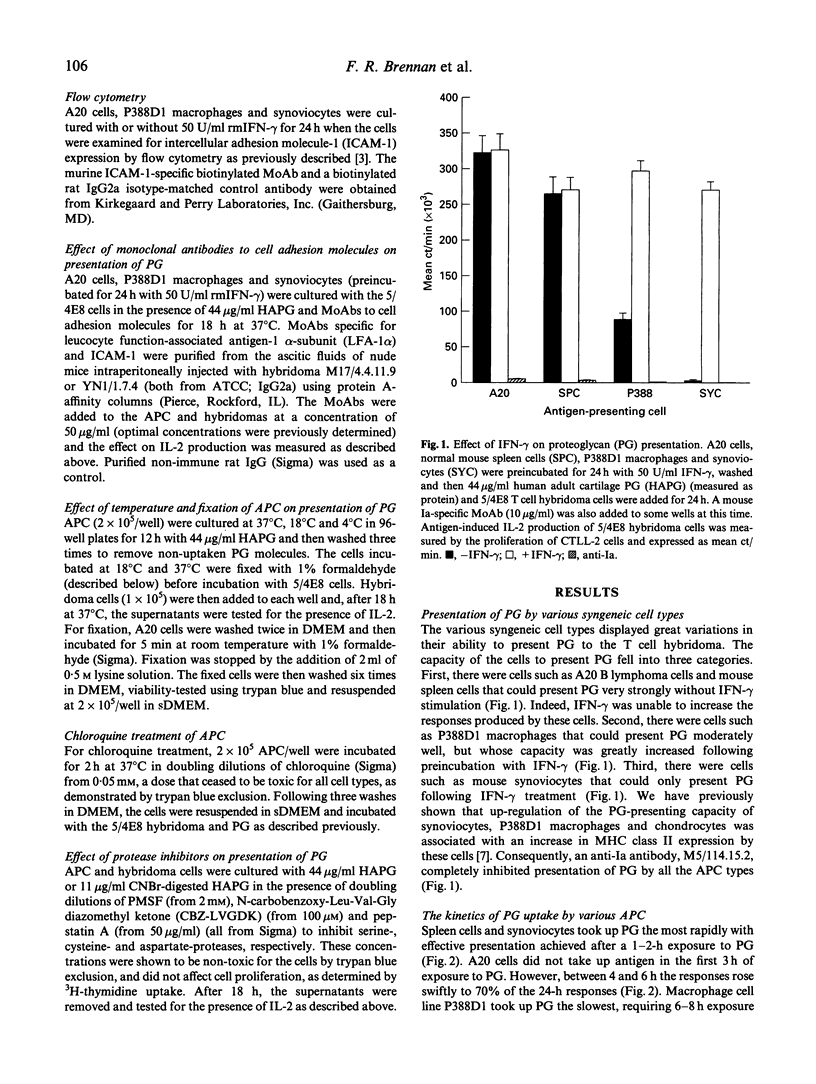
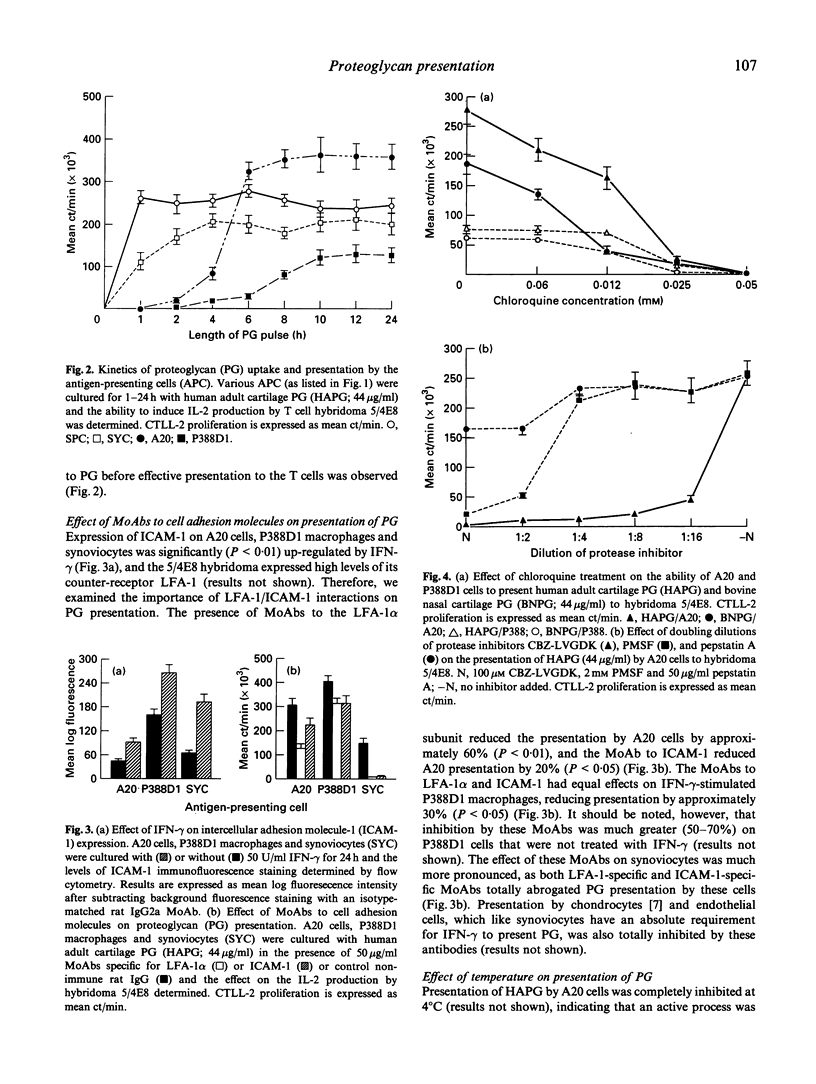
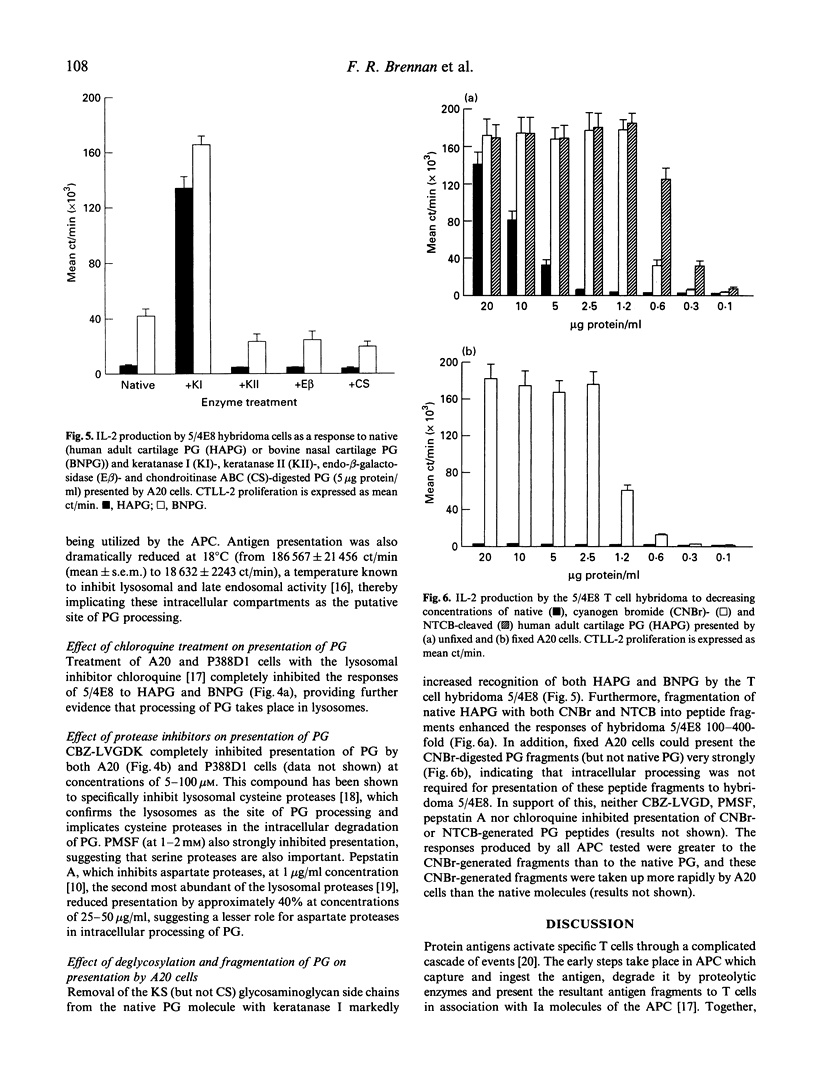
Immunization of BALB/c mice with human fetal cartilage proteoglycan (PG) produces progressive polyarthritis, and T cells play key roles in the development of the disease. To gain an understanding of how PG is presented to autoreactive T cells by synovial antigen-presenting cells (APC), we examined the abilities of various syngeneic APC in presenting PG to a specific T cell hybridoma 5/4E8, derived from a mouse with PG-induced arthritis. A20 B lymphoma cells and spleen cells were strong presenters of PG, but synoviocytes and P388D1 macrophages could only present PG effectively after stimulation with interferon-gamma (IFN-gamma). The IFN-gamma exerted its effect by up-regulating both MHC class II and intercellular adhesion molecule-1 (ICAM-1) expression by these cells as neutralizing antibodies to Ia, LFA-1 and ICAM-1 inhibited presentation. Our studies also showed that synoviocytes and spleen cells took up and processed PG more rapidly than the cell lines. Cysteine and serine protease-dependent antigen presentation of PG was blocked at 4 degrees C, 18 degrees C and by chloroquine treatment, indicating that presentation required active uptake and processing in an acidic compartment, probably in lysosomes. Also, keratan sulphate-depleted and cyanogen bromide (CNBr) and 2-nitro-5-thiocyanobenzoic acid (NTCB)-cleaved PG elicited stronger T cell responses, as they were more easily processed than the native molecule. Furthermore, CNBr-generated peptides were presented by fixed APC, indicating that core protein fragments of cartilage PG can be presented directly by APC in context with MHC class II molecules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buus S., Werdelin O. A group-specific inhibitor of lysosomal cysteine proteinases selectively inhibits both proteolytic degradation and presentation of the antigen dinitrophenyl-poly-L-lysine by guinea pig accessory cells to T cells. J Immunol. 1986 Jan;136(2):452–458. [PubMed] [Google Scholar]
- Buzás E. I., Mikecz K., Brennan F. R., Glant T. T. Mediators and autopathogenic effector cells in proteoglycan-induced arthritic and clinically asymptomatic BALB/c mice. Cell Immunol. 1994 Oct 15;158(2):292–304. doi: 10.1006/cimm.1994.1277. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Hardingham T. E. 1-C-6 epitope in cartilage proteoglycan G2 domain is masked by keratan sulphate. Biochem J. 1991 Jan 15;273(Pt 2):369–373. doi: 10.1042/bj2730369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Arzoumanian A., Poole A. R. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987 Feb;30(2):201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Poole A. R. Monoclonal antibodies to different protein-related epitopes of human articular cartilage proteoglycans. Biochem J. 1986 Feb 15;234(1):31–41. doi: 10.1042/bj2340031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golds E. E., Stephen I. B., Esdaile J. M., Strawczynski H., Poole A. R. Lymphocyte transformation to connective tissue antigens in adult and juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, systemic lupus erythematosus, and a nonarthritic control population. Cell Immunol. 1983 Nov;82(1):196–209. doi: 10.1016/0008-8749(83)90153-3. [DOI] [PubMed] [Google Scholar]
- Goodacre J. A., Middleton S., Lynn S., Ross D. A., Pearson J. Human cartilage aggrecan CS1 region contains cryptic T-cell recognition sites. Immunology. 1993 Apr;78(4):586–591. [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Low-temperature inhibition of antigen processing and iron uptake from transferrin: deficits in endosome functions at 18 degrees C. Eur J Immunol. 1990 Feb;20(2):323–329. doi: 10.1002/eji.1830200214. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Schaffer M. H., Stark G. R., Vanaman T. C. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973 Oct 10;248(19):6583–6591. [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T., Oppenheim J. J., Jasin H. E. Human interleukin 1 mediates cartilage matrix degradation. Cell Immunol. 1985 Mar;91(1):92–99. doi: 10.1016/0008-8749(85)90034-6. [DOI] [PubMed] [Google Scholar]
- Leyva-Cobian F., Unanue E. R. Intracellular interference with antigen presentation. J Immunol. 1988 Sep 1;141(5):1445–1450. [PubMed] [Google Scholar]
- Mikecz K., Glant T. T., Baron M., Poole A. R. Isolation of proteoglycan-specific T lymphocytes from patients with ankylosing spondylitis. Cell Immunol. 1988 Mar;112(1):55–63. doi: 10.1016/0008-8749(88)90275-4. [DOI] [PubMed] [Google Scholar]
- Mikecz K., Glant T. T., Poole A. R. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987 Mar;30(3):306–318. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Bressler L., Park L. S., Alpert A., Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987 Aug 15;139(4):1113–1119. [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. D., Firestein G. S., Taetle R., Kaushansky K., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989 Mar;83(3):876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., de Vries J. E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993 Jun 1;150(11):4754–4765. [PubMed] [Google Scholar]


