Abstract
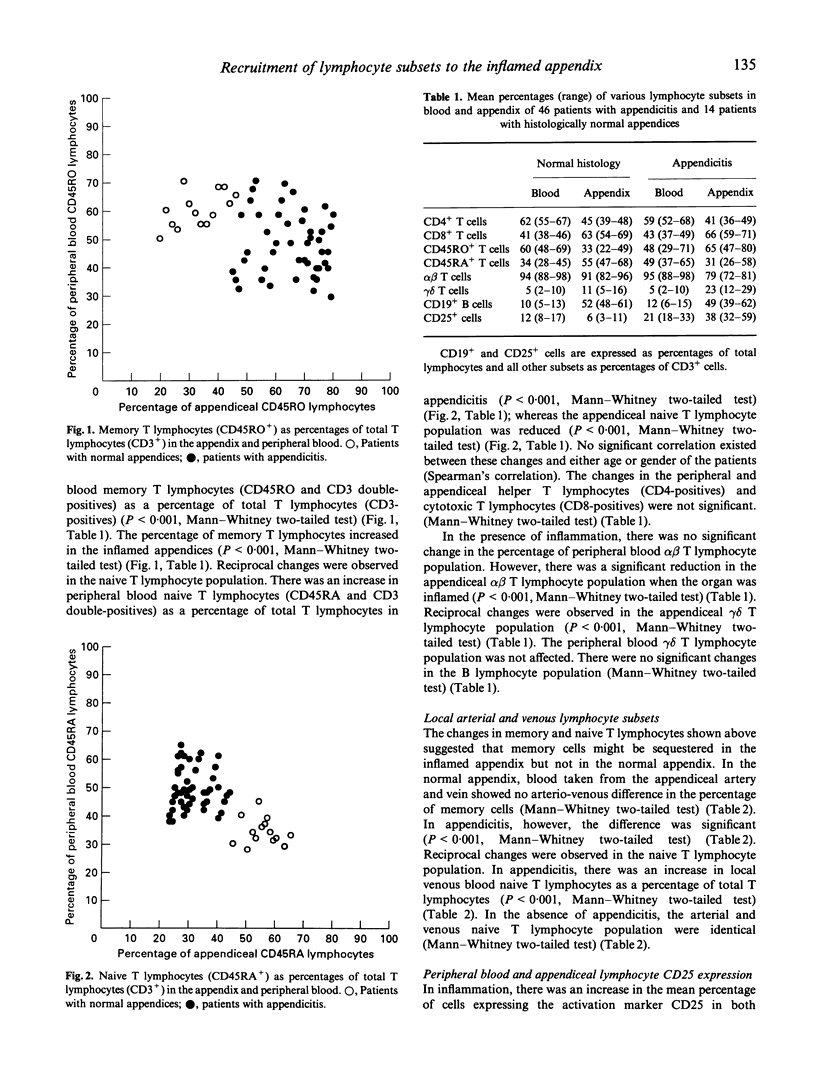
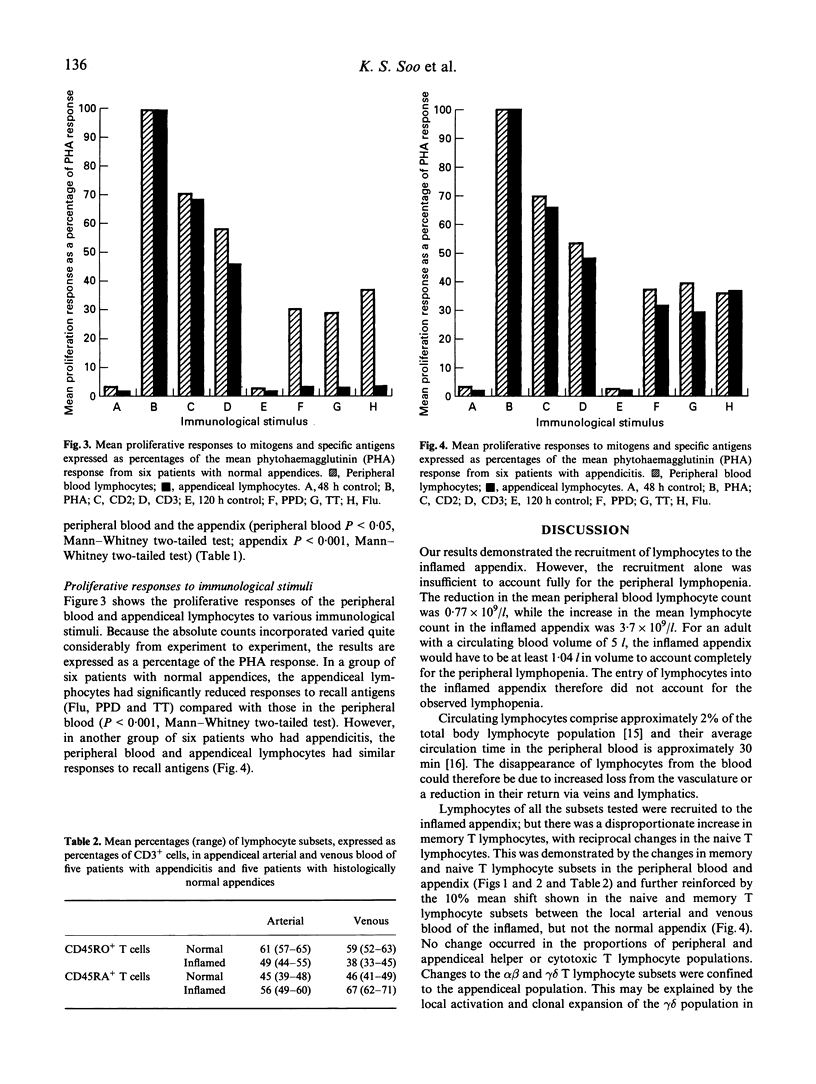
Total lymphocyte counts and the distribution of lymphocyte subsets were determined in peripheral venous blood and appendiceal mononuclear cells from 60 patients who underwent appendicectomy for the clinical diagnosis of appendicitis. A significant peripheral lymphopenia was observed in the 46 patients with histologically confirmed acute appendicitis which was accompanied by an increase in the appendiceal lymphocyte concentration. There was an even greater depletion of CD45RO+ (memory) T lymphocytes in peripheral blood and an increase in the inflamed appendix. Reciprocal changes were observed in the CD45RA+ (naive) T lymphocyte subset. These changes were reflected in the local arterial and venous CD45RA and CD45RO T lymphocyte subsets. Proliferation studies showed an expanded functional repertoire of T lymphocytes in the inflamed appendix. Selective recruitment of memory T lymphocytes from the peripheral blood to the inflamed appendix was demonstrated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Akbar A. N., Timms A., Janossy G. Cellular events during memory T-cell activation in vitro: the UCHL1 (180,000 MW) determinant is newly synthesized after mitosis. Immunology. 1989 Feb;66(2):213–218. [PMC free article] [PubMed] [Google Scholar]
- Baker S. R., Michie C. A., Soo K. S., Wyllie J. H., Beverley P. C. Lymphopenia in diverticulitis. Lancet. 1991 Aug 31;338(8766):569–570. doi: 10.1016/0140-6736(91)91131-d. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Borst J., van Dongen J. J., Bolhuis R. L., Peters P. J., Hafler D. A., de Vries E., van de Griend R. J. Distinct molecular forms of human T cell receptor gamma/delta detected on viable T cells by a monoclonal antibody. J Exp Med. 1988 May 1;167(5):1625–1644. doi: 10.1084/jem.167.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M. Growth of human T lymphocytes: an analysis of interleukin 2 and its cellular receptor. Prog Hematol. 1986;14:283–301. [PubMed] [Google Scholar]
- HERFORT K. F. Changes in the lymphocyte count during acute pancreatic necrosis. Acta Med Scand. 1950;137(2):97–103. doi: 10.1111/j.0954-6820.1950.tb11369.x. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Human CD8+ intraepithelial T lymphocytes are mainly CD45RA-RB+ and show increased co-expression of CD45R0 in celiac disease. Eur J Immunol. 1990 Aug;20(8):1825–1830. doi: 10.1002/eji.1830200829. [DOI] [PubMed] [Google Scholar]
- Healey D., Dianda L., Moore J. P., McDougal J. S., Moore M. J., Estess P., Buck D., Kwong P. D., Beverley P. C., Sattentau Q. J. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990 Oct 1;172(4):1233–1242. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet S., Wakasugi H., Sterkers G., Gilmour J., Tursz T., Boumsell L., Bernard A. T cell activation via CD2 [T, gp50]: the role of accessory cells in activating resting T cells via CD2. J Immunol. 1986 Sep 1;137(5):1420–1428. [PubMed] [Google Scholar]
- Jahangiri M., Wyllie J. H. Peripheral blood lymphopenia in gangrenous appendicitis. BMJ. 1990 Jul 28;301(6745):215–215. doi: 10.1136/bmj.301.6745.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988 Feb;9(2):43–45. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Terstappen L. W., Rott L. S., Streeter P. R., Stein H., Butcher E. C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990 Nov 15;145(10):3247–3255. [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G. H., Covelli M., Meliconi R., Markey A., Panayi G. S. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991 Feb;21(2):369–376. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Spits H., Borst J., Tax W., Capel P. J., Terhorst C., de Vries J. E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985 Sep;135(3):1922–1928. [PubMed] [Google Scholar]
- Sterry W., Bruhn S., Künne N., Lichtenberg B., Weber-Matthiesen K., Brasch J., Mielke V. Dominance of memory over naive T cells in contact dermatitis is due to differential tissue immigration. Br J Dermatol. 1990 Jul;123(1):59–64. doi: 10.1111/j.1365-2133.1990.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Westermann J., Pabst R. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today. 1990 Nov;11(11):406–410. doi: 10.1016/0167-5699(90)90160-b. [DOI] [PubMed] [Google Scholar]
- Zeitz M., Schieferdecker H. L., James S. P., Riecken E. O. Special functional features of T-lymphocyte subpopulations in the effector compartment of the intestinal mucosa and their relation to mucosal transformation. Digestion. 1990;46 (Suppl 2):280–289. doi: 10.1159/000200398. [DOI] [PubMed] [Google Scholar]


