Abstract
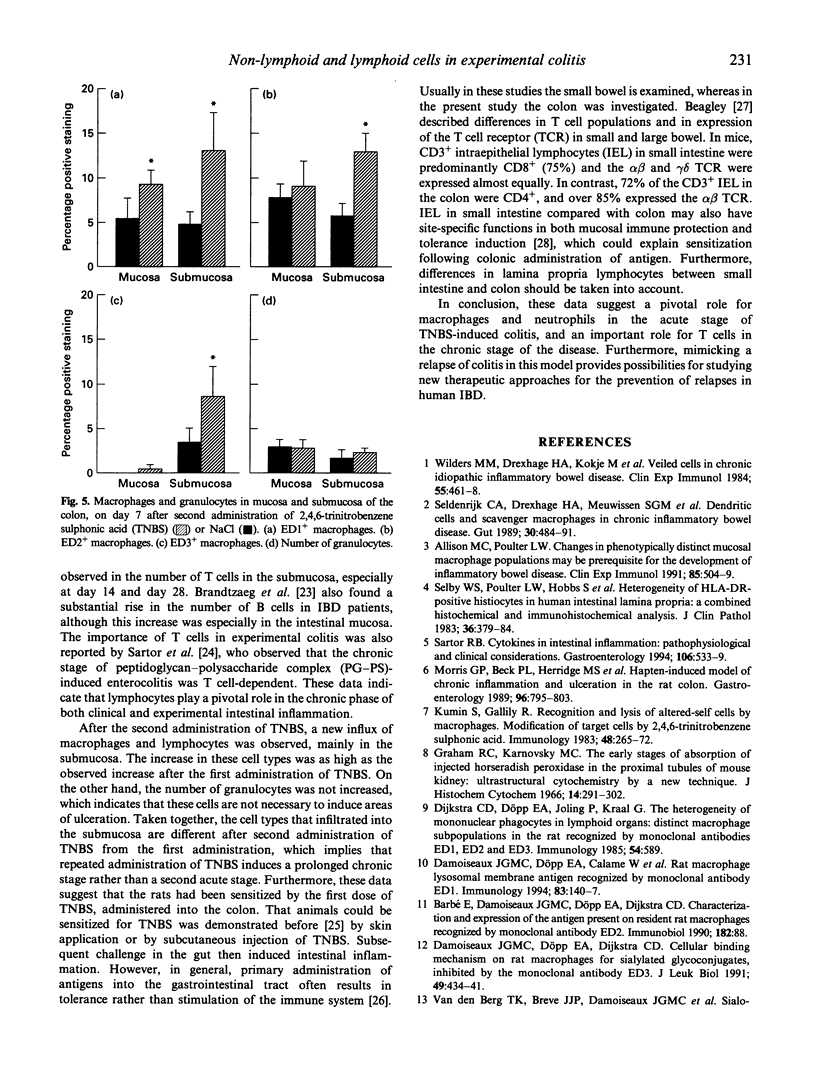
In rodents, intracolonic administration of ethanol 30% induces an acute colitis, while administration of 2,4,6-trinitrobenzene sulphonic acid (TNBS) in ethanol induces a longer lasting colitis. In the acute and chronic stages of experimental colitis, lymphoid and non-lymphoid cells were studied in the colon by immunohistochemistry. During the acute inflammation a high damage score of the colon was observed, which was related to an increase in the number of macrophages and granulocytes. Also a change in distributional patterns of macrophage subpopulations was found. The chronic stage of TNBS-ethanol-induced colitis was characterized by an increase in the number of lymphocytes, especially T cells. These data suggest that macrophages and granulocytes are important in the acute phase of experimental colitis, while lymphocytes play a pivotal role in the chronic stage. As most inflammatory bowel disease (IBD) patients have relapses during the chronic disease, we attempted to induce a relapse during experimental colitis by giving a second i.p. or s.c. dose of TNBS. This resulted in increased damage scores of the colon, new areas of ulceration and a further increase in macrophage numbers. No effect on the number of granulocytes was seen. These results indicate that it is possible to mimic relapses in experimental colitis by a second administration of TNBS, and suggest that the rats had been sensitized by the first dose of TNBS, given into the colon.
Full text
PDF

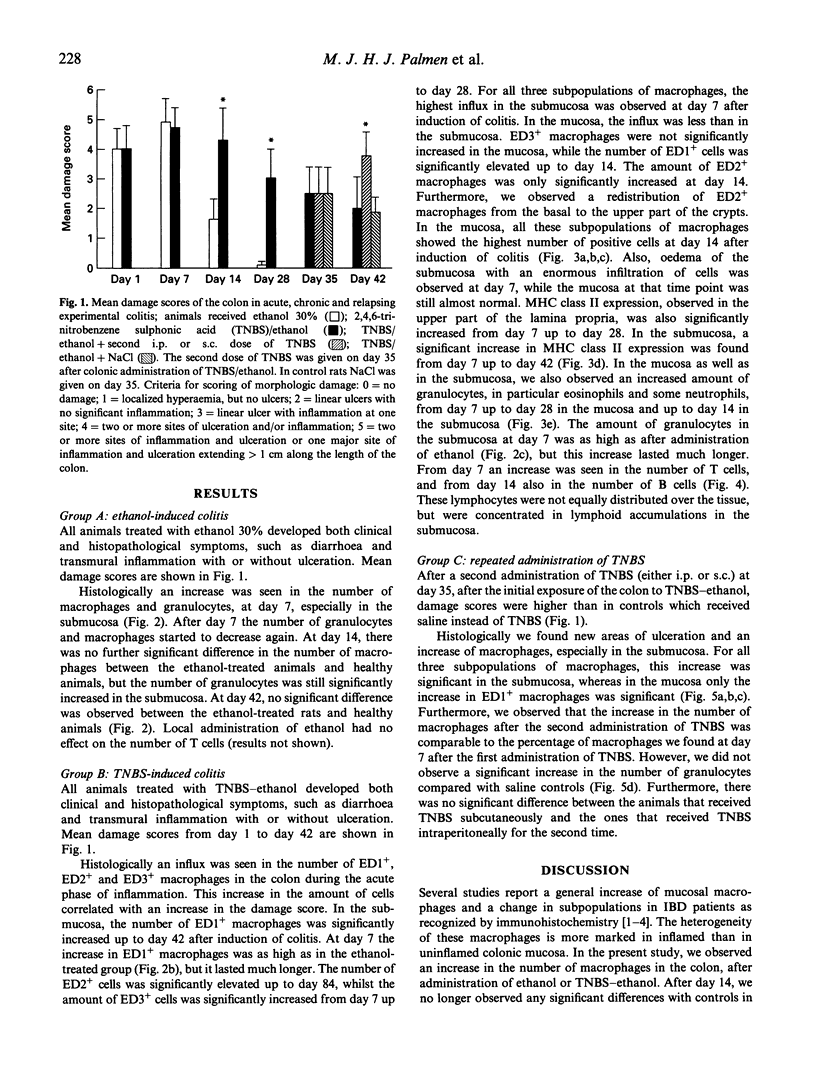

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Poulter L. W. Changes in phenotypically distinct mucosal macrophage populations may be a prerequisite for the development of inflammatory bowel disease. Clin Exp Immunol. 1991 Sep;85(3):504–509. doi: 10.1111/j.1365-2249.1991.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M., Dolezal I., Wolff K., Pehamberger H. Stereologic estimation of volume-weighted mean nuclear volume as a predictor of prognosis in "thin" malignant melanoma. J Invest Dermatol. 1992 Aug;99(2):180–183. doi: 10.1111/1523-1747.ep12616803. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Baklien K., Fausa O., Hoel P. S. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology. 1974 Jun;66(6):1123–1136. [PubMed] [Google Scholar]
- Camerini V., Panwala C., Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993 Aug 15;151(4):1765–1776. [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. G., Döpp E. A., Calame W., Chao D., MacPherson G. G., Dijkstra C. D. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994 Sep;83(1):140–147. [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J. G., Döpp E. A., Dijkstra C. D. Cellular binding mechanism on rat macrophages for sialylated glycoconjugates, inhibited by the monoclonal antibody ED3. J Leukoc Biol. 1991 May;49(5):434–441. doi: 10.1002/jlb.49.5.434. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
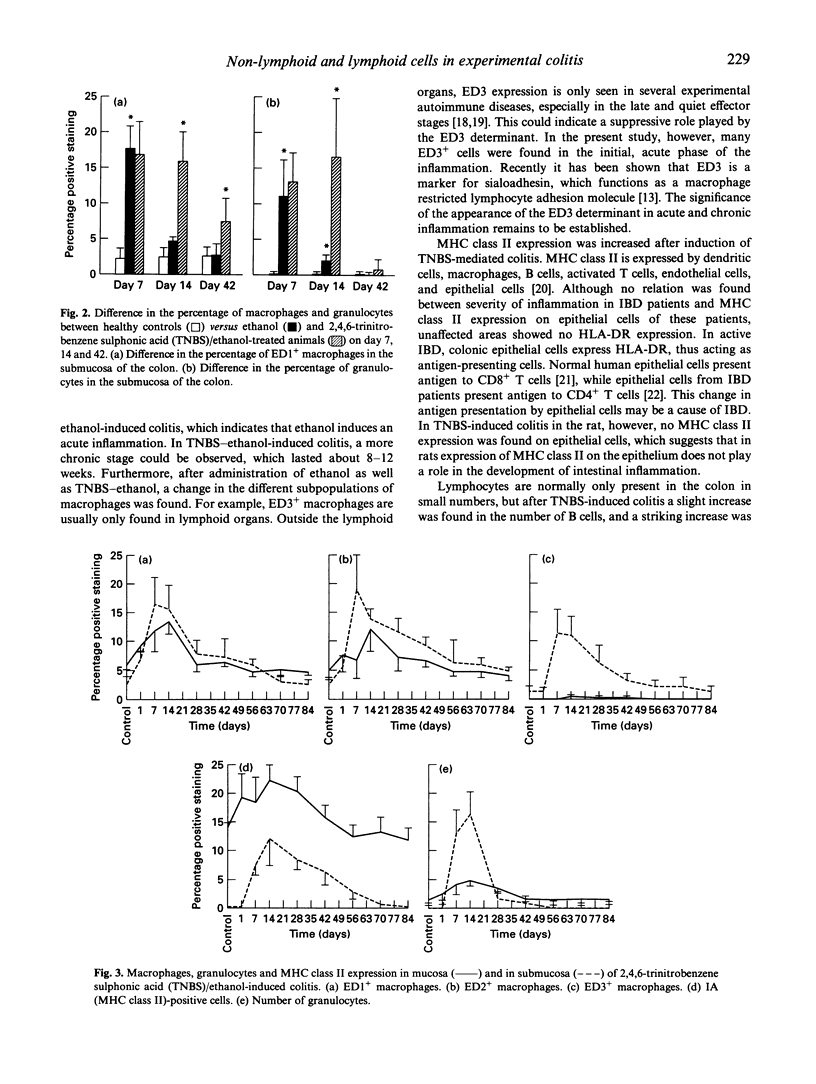
- Geboes K., Rutgeerts P., Ectors N., Mebis J., Penninckx F., Vantrappen G., Desmet V. J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology. 1992 Aug;103(2):439–447. doi: 10.1016/0016-5085(92)90832-j. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Kunin S., Gallily R. Recognition and lysis of altered-self cells by macrophages. I. Modification of target cells by 2,4,6-trinitrobenzene sulphonic acid. Immunology. 1983 Feb;48(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Eisenhardt D. Lack of induction of suppressor T cells by intestinal epithelial cells from patients with inflammatory bowel disease. J Clin Invest. 1990 Oct;86(4):1255–1260. doi: 10.1172/JCI114832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Noble B., Ren K., Taverne J., Dipirro J., Van Liew J., Dijkstra C., Janossy G., Poulter L. W. Mononuclear cells in glomeruli and cytokines in urine reflect the severity of experimental proliferative immune complex glomerulonephritis. Clin Exp Immunol. 1990 May;80(2):281–287. doi: 10.1111/j.1365-2249.1990.tb05248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Poulter L. W., Hobbs S., Jewell D. P., Janossy G. Heterogeneity of HLA-DR-positive histiocytes in human intestinal lamina propria: a combined histochemical and immunohistological analysis. J Clin Pathol. 1983 Apr;36(4):379–384. doi: 10.1136/jcp.36.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldenrijk C. A., Drexhage H. A., Meuwissen S. G., Pals S. T., Meijer C. J. Dendritic cells and scavenger macrophages in chronic inflammatory bowel disease. Gut. 1989 Apr;30(4):484–491. doi: 10.1136/gut.30.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure P. J., Van Noorden C. J., Dijkstra C. D. Macrophages and dendritic cells during the early stages of antigen-induced arthritis in rats: immunohistochemical analysis of cryostat sections of the whole knee joint. Scand J Immunol. 1989 Mar;29(3):371–381. doi: 10.1111/j.1365-3083.1989.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- van den Berg T. K., Brevé J. J., Damoiseaux J. G., Döpp E. A., Kelm S., Crocker P. R., Dijkstra C. D., Kraal G. Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J Exp Med. 1992 Sep 1;176(3):647–655. doi: 10.1084/jem.176.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]