Abstract
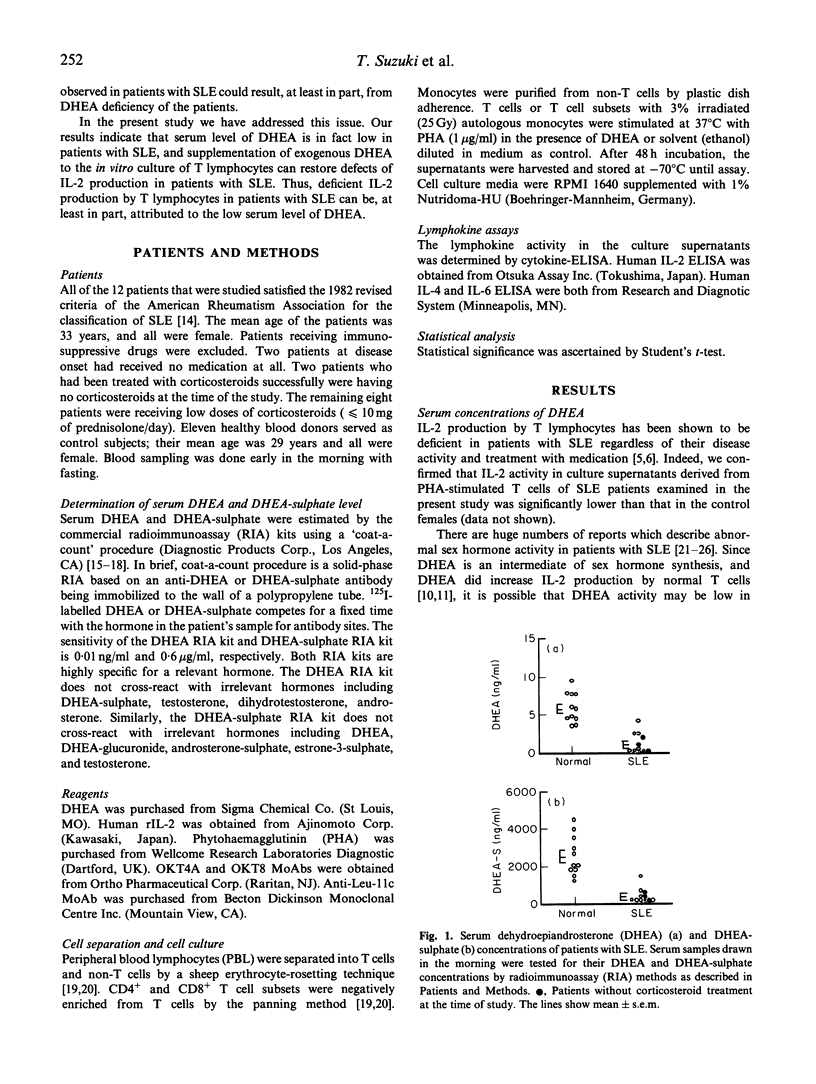
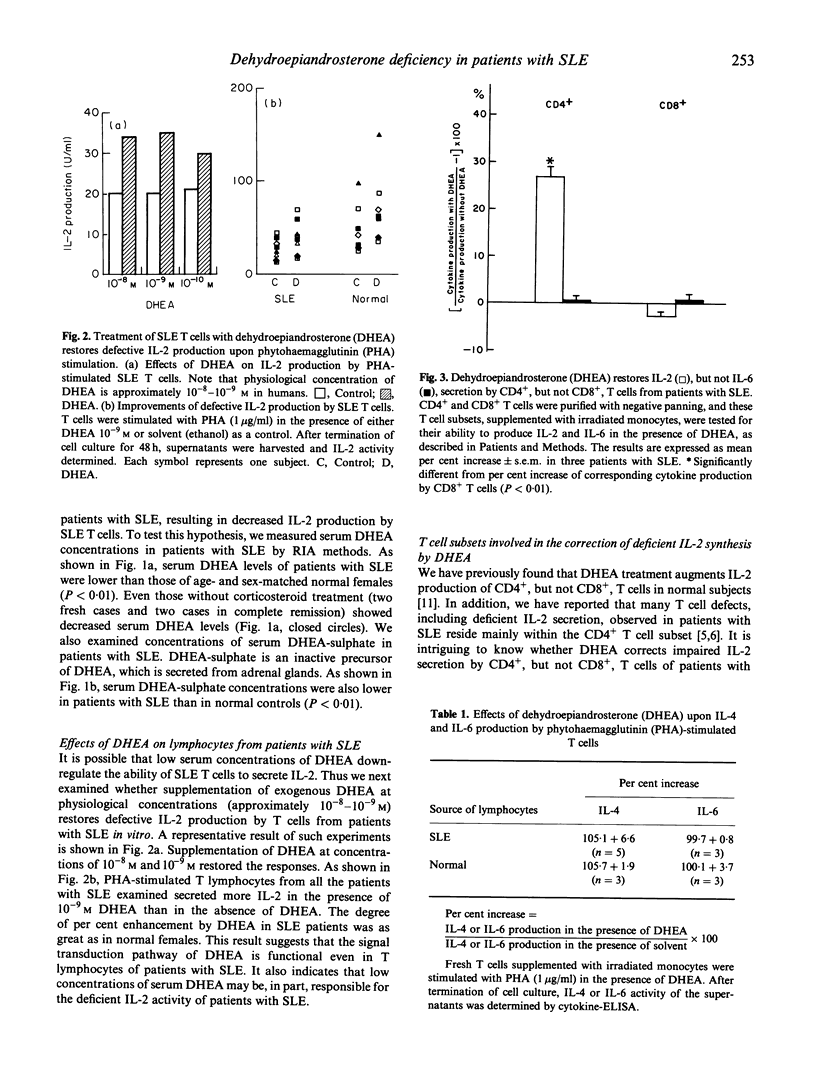
The principal cause of IL-2 deficiency, a common feature of both murine lupus and human SLE, remains obscure. Recent studies of our own as well as others have shown that dehydroepiandrosterone (DHEA), an intermediate compound in testosterone synthesis, significantly up-regulates IL-2 production of T cells, and that administration of exogenous DHEA or IL-2 via a vaccinia construct to murine lupus dramatically reverses their clinical autoimmune diseases. Thus, we have examined serum levels of DHEA in patients with SLE to test whether abnormal DHEA activity is associated with IL-2 deficiency of the patients. We found that nearly all of the patients examined have very low levels of serum DHEA. The decreased DHEA levels were not simply a reflection of a long term corticosteroid treatment which may cause adrenal atrophy, since serum samples drawn at the onset of disease, which are devoid of corticosteroid treatment, also contained low levels of DHEA. In addition, exogenous DHEA restored impaired IL-2 production of T cells from patients with SLE in vitro. These results indicate that defects of IL-2 synthesis of patients with SLE are at least in part due to the low DHEA activity in the serum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aakvaag A., Fylling P. A method for the simultaneous determination of progesterone, androstenedione, testosterone and dehydroepiandrosterone sulphate in biological fluids. Its application in the analysis of venous plasma and cyst fluid from human ovaries in situ. Acta Endocrinol (Copenh) 1968 Mar;57(3):447–456. doi: 10.1530/acta.0.0570447. [DOI] [PubMed] [Google Scholar]
- Buyalos R. P., Bradley E. L., Jr, Judd H. L., Zacur H. A., Azziz R. No acute effect of physiological insulin increase on dehydroepiandrosterone sulfate in women with obesity and/or polycystic ovarian disease. Fertil Steril. 1991 Dec;56(6):1179–1182. [PubMed] [Google Scholar]
- Cutolo M., Balleari E., Giusti M., Intra E., Accardo S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 1991 Jan;34(1):1–5. doi: 10.1002/art.1780340102. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Gooren L., Spinder T., Spijkstra J. J., van Kessel H., Smals A., Rao B. R., Hoogslag M. Sex steroids and pulsatile luteinizing hormone release in men. Studies in estrogen-treated agonadal subjects and eugonadal subjects treated with a novel nonsteroidal antiandrogen. J Clin Endocrinol Metab. 1987 Apr;64(4):763–770. doi: 10.1210/jcem-64-4-763. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Shantz L. M., Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ramos J. C., Andreu J. L., Revilla Y., Viñuela E., Martinez C. Recovery from autoimmunity of MRL/lpr mice after infection with an interleukin-2/vaccinia recombinant virus. Nature. 1990 Jul 19;346(6281):271–274. doi: 10.1038/346271a0. [DOI] [PubMed] [Google Scholar]
- Hall G. M., Perry L. A., Spector T. D. Depressed levels of dehydroepiandrosterone sulphate in postmenopausal women with rheumatoid arthritis but no relation with axial bone density. Ann Rheum Dis. 1993 Mar;52(3):211–214. doi: 10.1136/ard.52.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman M., Nilsson E., de la Torre B. Low blood and synovial fluid levels of sulpho-conjugated steroids in rheumatoid arthritis. Clin Exp Rheumatol. 1992 Jan-Feb;10(1):25–30. [PubMed] [Google Scholar]
- Hedman M., Nilsson E., de la Torre B. Low sulpho-conjugated steroid hormone levels in systemic lupus erythematosus (SLE). Clin Exp Rheumatol. 1989 Nov-Dec;7(6):583–588. [PubMed] [Google Scholar]
- Jungers P., Kuttenn F., Liote F., Pelissier C., Athea N., Laurent M. C., Viriot J., Dougados M., Bach J. F. Hormonal modulation in systemic lupus erythematosus. Preliminary clinical and hormonal results with cyproterone acetate. Arthritis Rheum. 1985 Nov;28(11):1243–1250. doi: 10.1002/art.1780281108. [DOI] [PubMed] [Google Scholar]
- Jungers P., Nahoul K., Pelissier C., Dougados M., Tron F., Bach J. F. Low plasma androgens in women with active or quiescent systemic lupus erythematosus. Arthritis Rheum. 1982 Apr;25(4):454–457. doi: 10.1002/art.1780250415. [DOI] [PubMed] [Google Scholar]
- Lahita R. G., Bradlow H. L., Ginzler E., Pang S., New M. Low plasma androgens in women with systemic lupus erythematosus. Arthritis Rheum. 1987 Mar;30(3):241–248. doi: 10.1002/art.1780300301. [DOI] [PubMed] [Google Scholar]
- Lahita R. G., Kunkel H. G., Bradlow H. L. Increased oxidation of testosterone in systemic lupus erythematosus. Arthritis Rheum. 1983 Dec;26(12):1517–1521. doi: 10.1002/art.1780261215. [DOI] [PubMed] [Google Scholar]
- Loucks A. B., Horvath S. M. Exercise-induced stress responses of amenorrheic and eumenorrheic runners. J Clin Endocrinol Metab. 1984 Dec;59(6):1109–1120. doi: 10.1210/jcem-59-6-1109. [DOI] [PubMed] [Google Scholar]
- Lucas J. A., Ahmed S. A., Casey M. L., MacDonald P. C. Prevention of autoantibody formation and prolonged survival in New Zealand black/New Zealand white F1 mice fed dehydroisoandrosterone. J Clin Invest. 1985 Jun;75(6):2091–2093. doi: 10.1172/JCI111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. H., Urowitz M. B., Gladman D. D., Killinger D. W. Systemic lupus erythematosus in males. Medicine (Baltimore) 1983 Sep;62(5):327–334. doi: 10.1097/00005792-198309000-00005. [DOI] [PubMed] [Google Scholar]
- Murakawa Y., Sakane T. Deficient phytohemagglutinin-induced interleukin-2 activity in patients with inactive systemic lupus erythematosus is correctable by the addition of phorbol myristate acetate. Arthritis Rheum. 1988 Jul;31(7):826–833. doi: 10.1002/art.1780310702. [DOI] [PubMed] [Google Scholar]
- Murakawa Y., Takada S., Ueda Y., Suzuki N., Hoshino T., Sakane T. Characterization of T lymphocyte subpopulations responsible for deficient interleukin 2 activity in patients with systemic lupus erythematosus. J Immunol. 1985 Jan;134(1):187–195. [PubMed] [Google Scholar]
- Nagafuchi H., Suzuki N., Mizushima Y., Sakane T. Constitutive expression of IL-6 receptors and their role in the excessive B cell function in patients with systemic lupus erythematosus. J Immunol. 1993 Dec 1;151(11):6525–6534. [PubMed] [Google Scholar]
- Sakane T., Murakawa Y., Suzuki N., Ueda Y., Tsuchida T., Takada S., Yamauchi Y., Tsunematsu T. Familial occurrence of impaired interleukin-2 activity and increased peripheral blood B cells actively secreting immunoglobulins in systemic lupus erythematosus. Am J Med. 1989 Apr;86(4):385–390. doi: 10.1016/0002-9343(89)90334-3. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Lowest dose interleukin-2 immunotherapy. Blood. 1993 Mar 15;81(6):1414–1423. [PubMed] [Google Scholar]
- Suzuki N., Sakane T., Engleman E. G. Anti-DNA antibody production by CD5+ and CD5- B cells of patients with systemic lupus erythematosus. J Clin Invest. 1990 Jan;85(1):238–247. doi: 10.1172/JCI114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Suzuki N., Daynes R. A., Engleman E. G. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):202–211. doi: 10.1016/s0090-1229(05)80024-8. [DOI] [PubMed] [Google Scholar]
- Takada S., Ueda Y., Suzuki N., Murakawa Y., Hoshino T., Green I., Steinberg A. D., Horwitz D. A., Sakane T. Abnormalities in autologous mixed lymphocyte reaction-activated immunologic processes in systemic lupus erythematosus and their possible correction by interleukin 2. Eur J Immunol. 1985 Mar;15(3):262–267. doi: 10.1002/eji.1830150310. [DOI] [PubMed] [Google Scholar]
- Talal N., Dauphinee M. J., Wofsy D. Interleukin-2 deficiency, genes, and systemic lupus erythematosus. Arthritis Rheum. 1982 Jul;25(7):838–842. doi: 10.1002/art.1780250725. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]


