Abstract
The protozoan parasite of cattle, Theileria annulata, causes a severe lymphoproliferative disease, developing initially in the draining lymph node, which is often fatal in naive animals. Infection of macrophages with T. annulata leads to an augmentation of their antigen-presenting capability in vitro and infected cells can induce proliferation of autologous resting T cells from naive animals. This inappropriate activation of T cells may play an important role in the failure of the host to mount an effective immune response in vivo. To investigate this hypothesis we characterized further the response of T cells from naive cattle to infected cells in vitro, and also examined the development of the immune response in lymph nodes draining the sites of T. annulata infection. Both CD4+ and CD8+ T cells from naive peripheral blood mononuclear cells (PBMC) were induced to proliferate and express the activation markers IL-2R and MHC class II when cultured with infected cells. This effect was seen in both 'naive' and 'memory' T cells, and was dependent upon contact with infected cells. In vitro, infected cells are therefore capable of activating T cells irrespective of their antigen specificity or memory status. In draining lymph nodes, although large numbers of IL-2R+ cells developed following infection, these activated cells were only associated with areas of parasite-induced proliferating cells, and subsequently disappeared from the node. Cells expressing IL-2R were not present in recognized sites for T cell development. Germinal centres were severely affected, losing T cell-dependent zones followed by a total destruction of morphology. T cell function is therefore severely disrupted within draining nodes. This study has shown that parasitized cells supply sufficient signals in vitro to activate T cells irrespective of specificity. T cells also are not stimulated in a conventional manner in vivo, and this may play an important role in preventing an effective immune response from being generated.
Full text
PDF

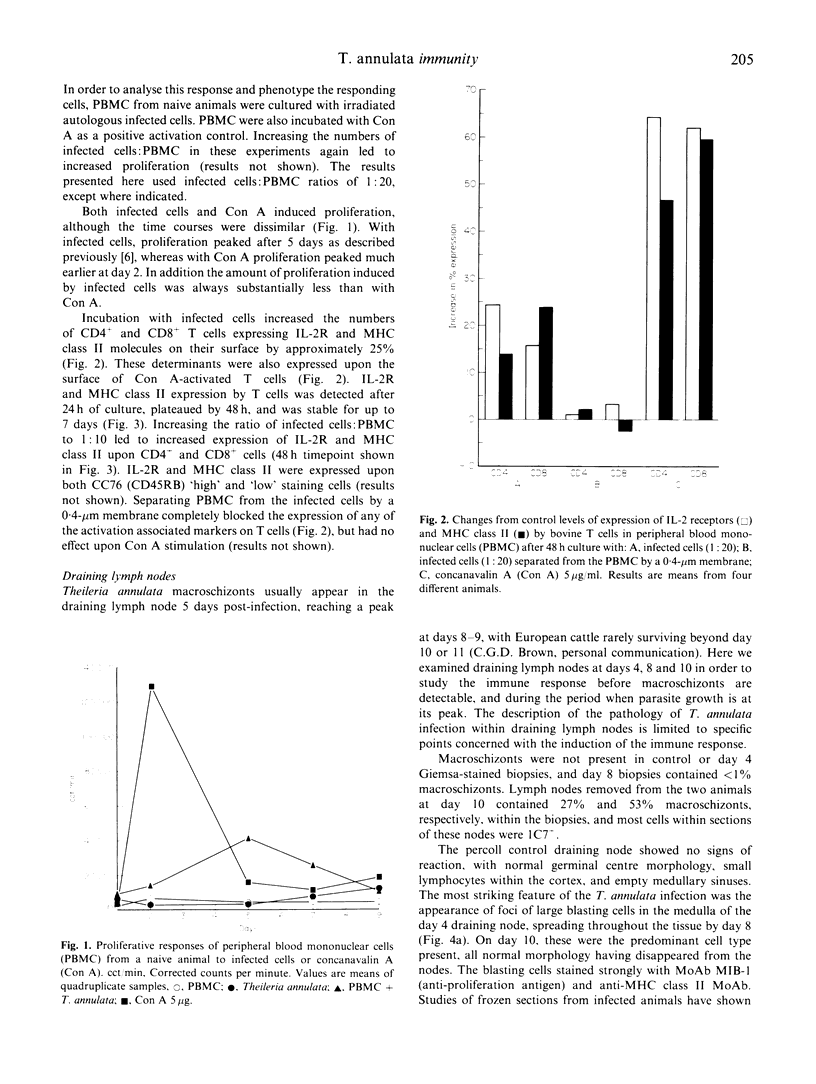
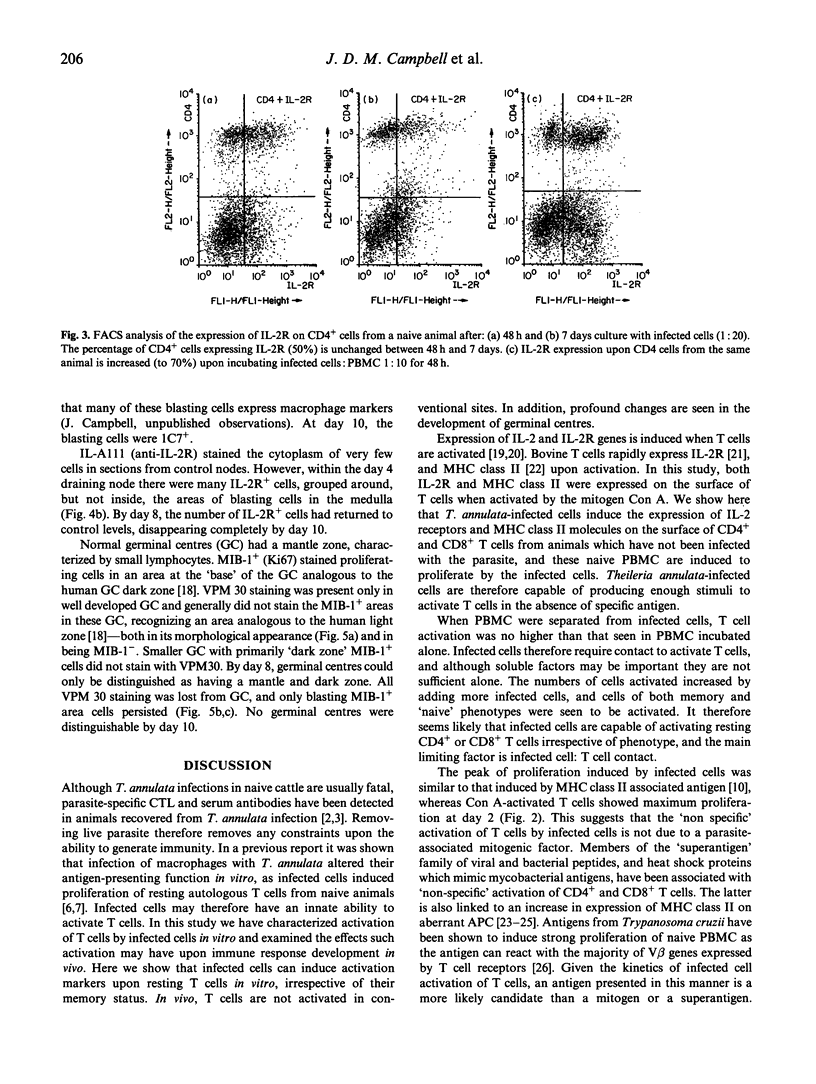
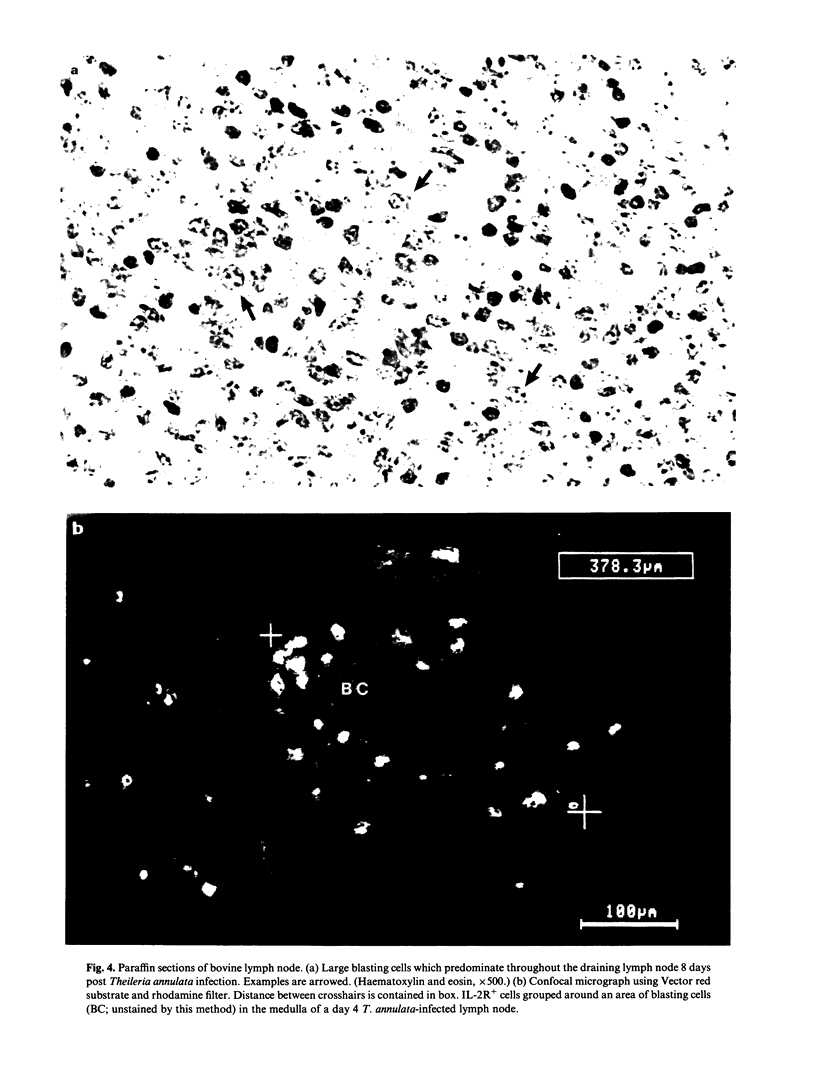
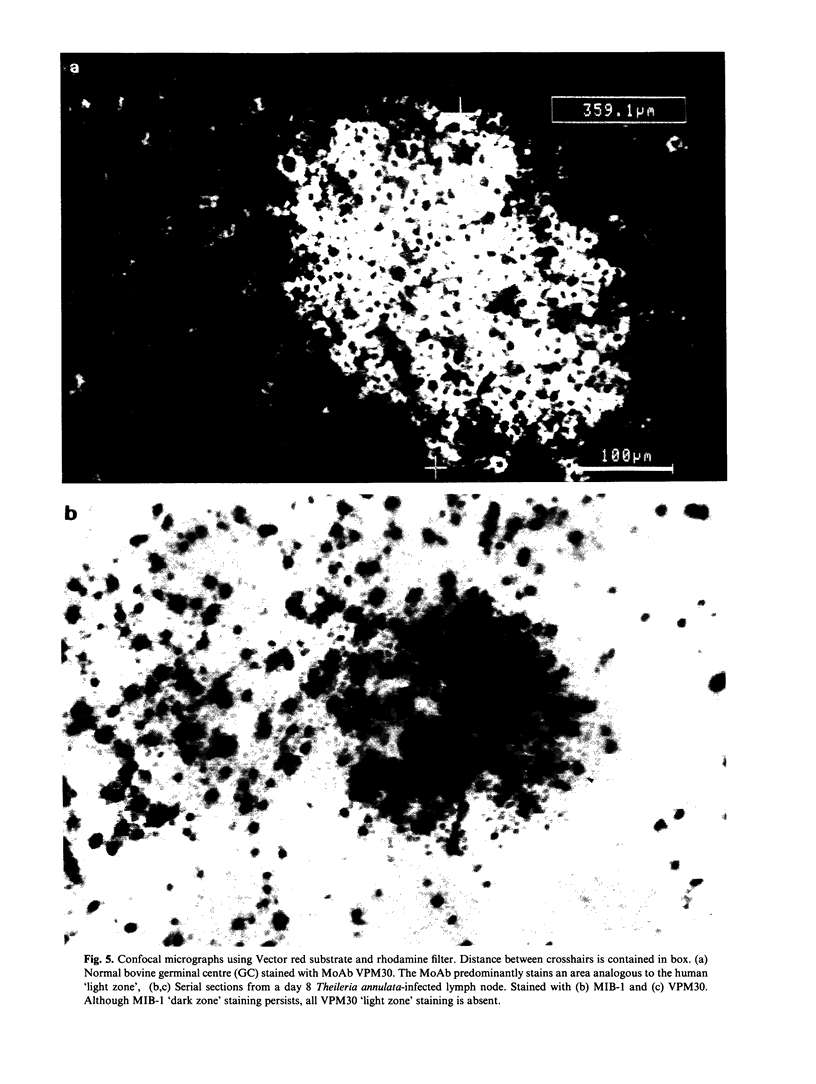


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Teale A. J., Naessens J. G., Goddeeris B. M., MacHugh N. D., Morrison W. I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986 Jun 15;136(12):4385–4391. [PubMed] [Google Scholar]
- Bogen S. A., Weinberg D. S., Abbas A. K. Histologic analysis of T lymphocyte activation in reactive lymph nodes. J Immunol. 1991 Sep 1;147(5):1537–1541. [PubMed] [Google Scholar]
- Dhar S., Malhotra D. V., Bhushan C., Gautam O. P. Chemoimmunoprophylaxis against bovine tropical theileriosis in young calves: a comparison between buparvaquone and long-acting oxytetracycline. Res Vet Sci. 1990 Jul;49(1):110–112. [PubMed] [Google Scholar]
- Glass E. J., Innes E. A., Spooner R. L., Brown C. G. Infection of bovine monocyte/macrophage populations with Theileria annulata and Theileria parva. Vet Immunol Immunopathol. 1989 Nov 15;22(4):355–368. doi: 10.1016/0165-2427(89)90171-2. [DOI] [PubMed] [Google Scholar]
- Glass E. J., Spooner R. L. Generation and characterisation of bovine antigen-specific T cell lines. J Immunol Methods. 1990 Apr 17;128(2):267–275. doi: 10.1016/0022-1759(90)90219-l. [DOI] [PubMed] [Google Scholar]
- Glass E. J., Spooner R. L. Parasite-accessory cell interactions in theileriosis. Antigen presentation by Theileria annulata-infected macrophages and production of continuously growing antigen-presenting cell lines. Eur J Immunol. 1990 Nov;20(11):2491–2497. doi: 10.1002/eji.1830201120. [DOI] [PubMed] [Google Scholar]
- Glass E. J., Spooner R. L. Requirement for MHC class II positive accessory cells in an antigen specific bovine T cell response. Res Vet Sci. 1989 Mar;46(2):196–201. [PubMed] [Google Scholar]
- Hardie D. L., Johnson G. D., Khan M., MacLennan I. C. Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur J Immunol. 1993 May;23(5):997–1004. doi: 10.1002/eji.1830230502. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Sopp P., Parsons K. R., McKeever D. J., Taracha E. L., Jones B. V., MacHugh N. D., Morrison W. I. Distinction of naive and memory BoCD4 lymphocytes in calves with a monoclonal antibody, CC76, to a restricted determinant of the bovine leukocyte-common antigen, CD45. Eur J Immunol. 1991 Sep;21(9):2219–2226. doi: 10.1002/eji.1830210933. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Deulofeut H., Dubey D. P., Salazar M., Egea E., Yunis E. J., Fraser P. A. Functional deficiency of antigen-presenting cells in the synovial fluid of rheumatoid arthritis. Hum Immunol. 1992 Oct;35(2):109–115. doi: 10.1016/0198-8859(92)90018-i. [DOI] [PubMed] [Google Scholar]
- Innes E. A., Millar P., Brown C. G., Spooner R. L. The development and specificity of cytotoxic cells in cattle immunized with autologous or allogeneic Theileria annulata-infected lymphoblastoid cell lines. Parasite Immunol. 1989 Jan;11(1):57–68. doi: 10.1111/j.1365-3024.1989.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Jacobson E. B., Caporale L. H., Thorbecke G. J. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell Immunol. 1974 Sep;13(3):416–430. doi: 10.1016/0008-8749(74)90261-5. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Key G., Becker M. H., Baron B., Duchrow M., Schlüter C., Flad H. D., Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993 Jun;68(6):629–636. [PubMed] [Google Scholar]
- MacLennan I. C. Somatic mutation. From the dark zone to the light. Curr Biol. 1994 Jan 1;4(1):70–72. doi: 10.1016/s0960-9822(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- McKeever D. J., Awino E., Morrison W. I. Afferent lymph veiled cells prime CD4+ T cell responses in vivo. Eur J Immunol. 1992 Dec;22(12):3057–3061. doi: 10.1002/eji.1830221205. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naessens J., Howard C. J. Individual antigens of cattle. Monoclonal antibodies reacting with bovine B cells (BoWC3, BoWC4 and BoWC5). Vet Immunol Immunopathol. 1991 Jan;27(1-3):77–85. doi: 10.1016/0165-2427(91)90083-o. [DOI] [PubMed] [Google Scholar]
- Naessens J., Sileghem M., MacHugh N., Park Y. H., Davis W. C., Toye P. Selection of BoCD25 monoclonal antibodies by screening mouse L cells transfected with the bovine p55-interleukin-2 (IL-2) receptor gene. Immunology. 1992 Jun;76(2):305–309. [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis P., Kroese F. G., Opstelten D., Seijen H. G. De novo germinal center formation. Immunol Rev. 1992 Apr;126:77–98. doi: 10.1111/j.1600-065x.1992.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Piuvezam M. R., Russo D. M., Burns J. M., Jr, Skeiky Y. A., Grabstein K. H., Reed S. G. Characterization of responses of normal human T cells to Trypanosoma cruzi antigens. J Immunol. 1993 Feb 1;150(3):916–924. [PubMed] [Google Scholar]
- Preston P. M., Brown C. G., Spooner R. L. Cell-mediated cytotoxicity in Theileria annulata infection of cattle with evidence for BoLA restriction. Clin Exp Immunol. 1983 Jul;53(1):88–100. [PMC free article] [PubMed] [Google Scholar]
- Rintelen M., Schein E., Ahmed J. S. Buparvaquone but not cyclosporin A prevents Theileria annulata-infected bovine lymphoblastoid cells from stimulating uninfected lymphocytes. Trop Med Parasitol. 1990 Jun;41(2):203–207. [PubMed] [Google Scholar]
- Shiels B. R., McDougall C., Tait A., Brown C. G. Identification of infection-associated antigens in Theileria annulata transformed cells. Parasite Immunol. 1986 Jan;8(1):69–77. doi: 10.1111/j.1365-3024.1986.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Siess D. C., Magnuson N. S., Reeves R. Characterization of the bovine receptor(s) for interleukin-2. Immunology. 1989 Oct;68(2):190–195. [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Spooner R. L., Innes E. A., Glass E. J., Brown C. G. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology. 1989 Feb;66(2):284–288. [PMC free article] [PubMed] [Google Scholar]
- Walker A. R., McKellar S. B. Observations on the separation of Theileria sporozoites from ticks. Int J Parasitol. 1983 Jun;13(3):313–318. doi: 10.1016/0020-7519(83)90044-9. [DOI] [PubMed] [Google Scholar]





