Abstract
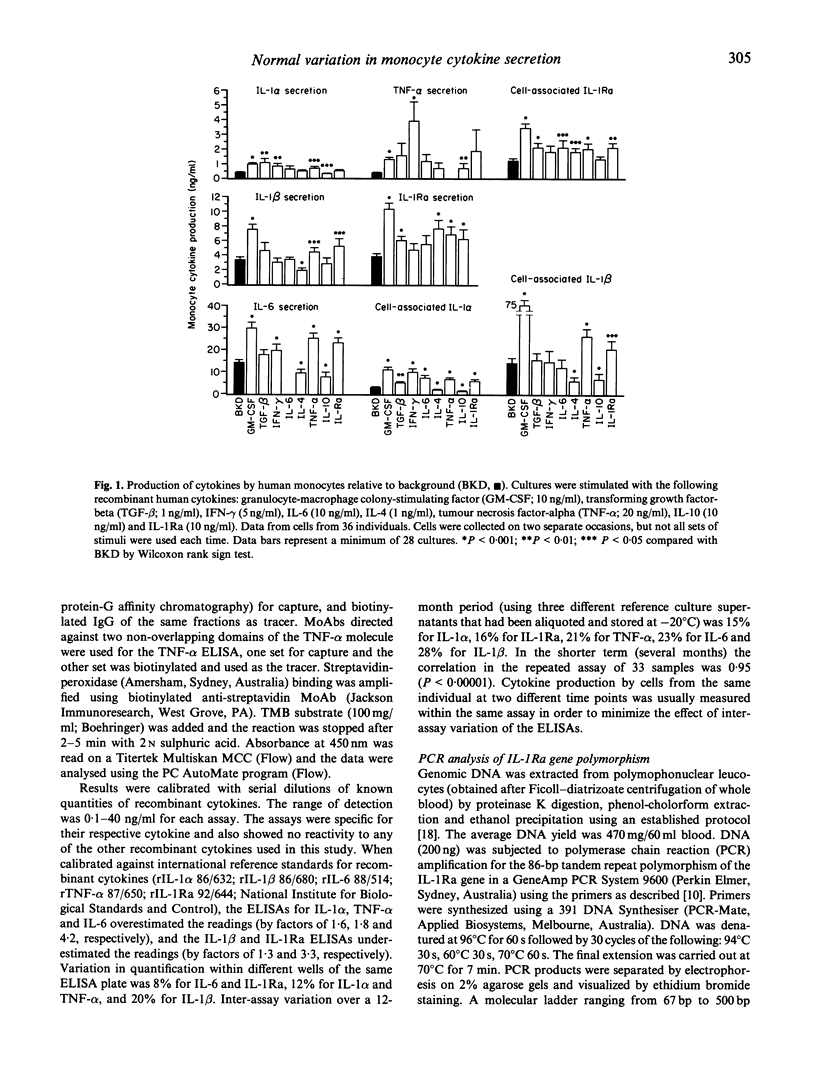
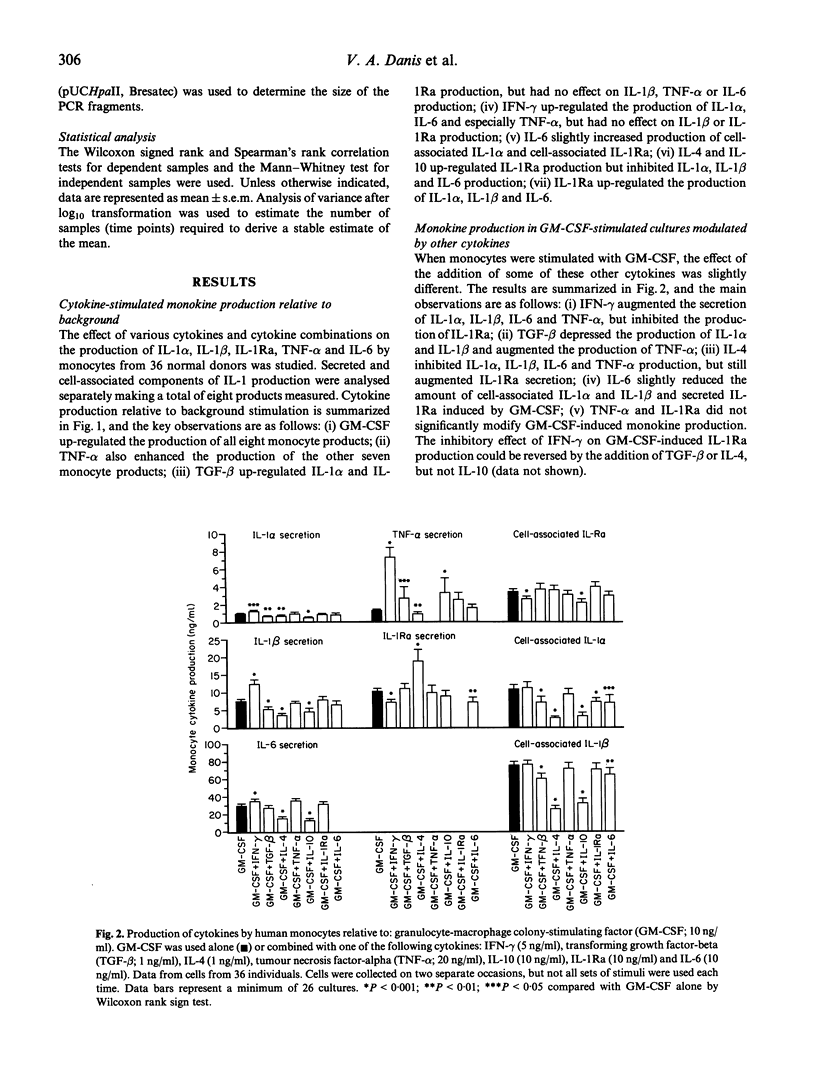
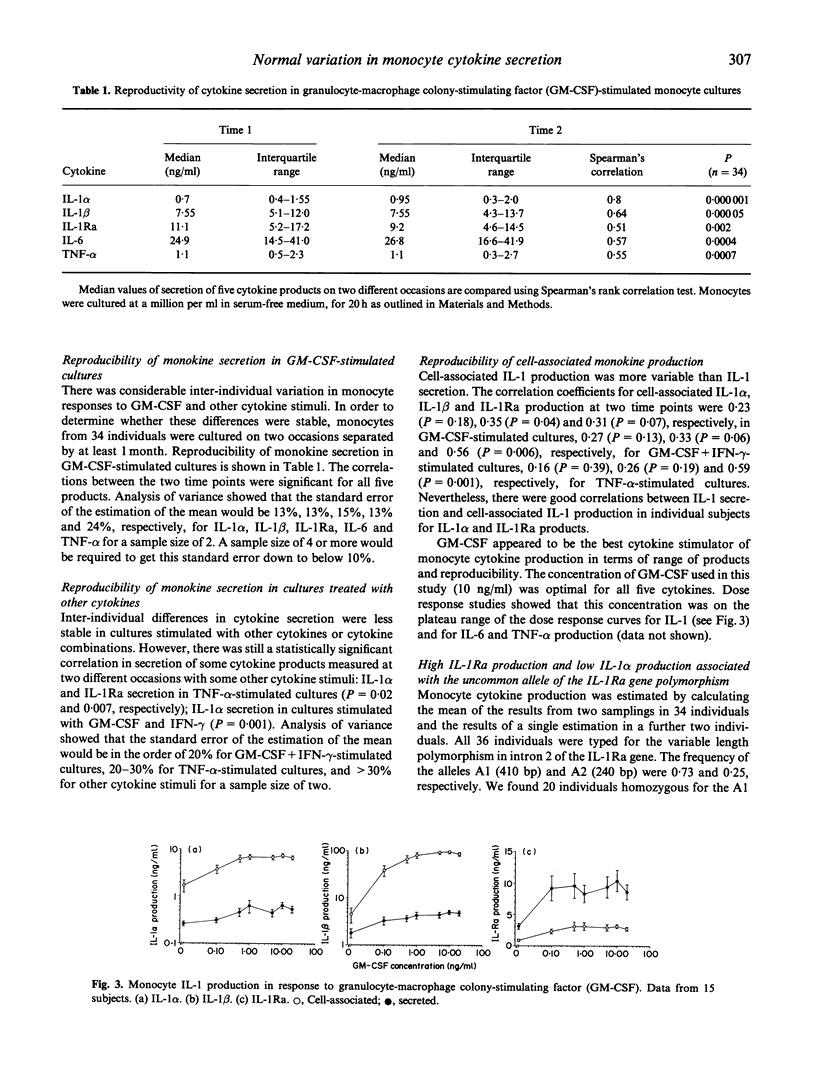
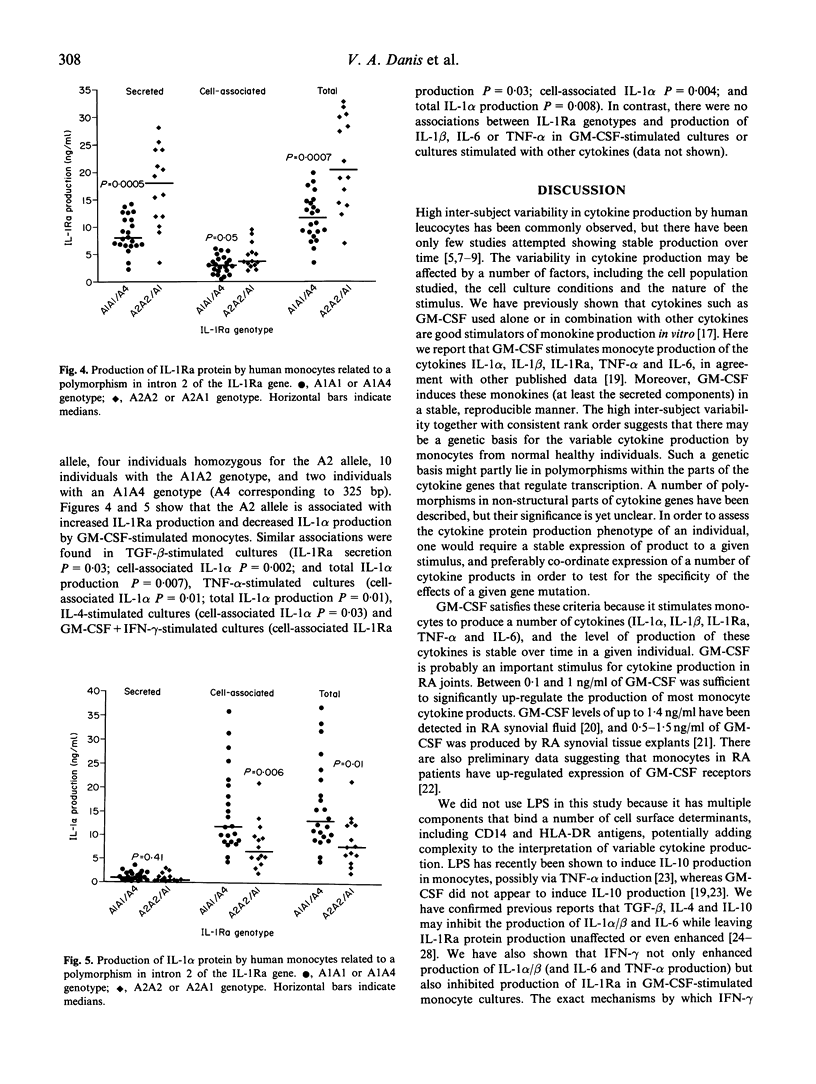
Monocytes from different individuals show variable cytokine production in response to a variety of stimuli. We wished to determine the sets of conditions (cytokine combinations) that would enable us to demonstrate stable inter-individual differences in the production of IL-1 alpha, IL-1 beta, IL-1Ra, on-6 and tumour necrosis factor-alpha (TNF-alpha) by monocytes. We assessed the ability of a number of recombinant human cytokines (granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-gamma), TNF-alpha, IL-4, IL-6, transforming growth factor-beta (TGF-beta), IL-10 and IL-1Ra)) to stimulate or inhibit the production of one or more of these monocyte products. GM-CSF was found to stimulate the production of all five of these cytokines in a highly reproducible manner. TNF-alpha also up-regulated production of IL-1 alpha, IL-1 beta, IL-1Ra and IL-6 by monocytes, but the variability in the results of cells cultured from the same individuals on different occasions was greater. Other cytokines either stimulated production of only some of the five cytokine products tested, or stimulated the production of some cytokine products while inhibiting production of others. This was especially evident when cytokines were used in combination with GM-CSF: IFN-gamma down-regulated production of IL-1Ra while up-regulating the production of IL-1 alpha/beta, IL-6 and TNF-alpha, while IL-4 had the exact opposite effect. Polymorphisms in regions of cytokine genes that affect transcription may account for some of the interindividual variation in cytokine production. We have shown that a stable estimate of cytokine production phenotype can be obtained when monocytes collected on at least two separate occasions are stimulated by GM-CSF in vitro. We have looked for a relationship between IL-1 production and an 86-bp variable repeat polymorphism in intron 2 of the IL-1Ra gene. A less common allele of this polymorphism (allele 2) was associated with increased production of IL-1Ra protein, and also reduced production of IL-1 alpha protein by monocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly S., di Giovine F. S., Blakemore A. I., Duff G. W. Genetic polymorphism of human interleukin-1 alpha. Eur J Immunol. 1993 Jun;23(6):1240–1245. doi: 10.1002/eji.1830230607. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Paik J., Vodovotz Y., Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992 Nov 15;267(32):23301–23308. [PubMed] [Google Scholar]
- Cluitmans F. H., Esendam B. H., Landegent J. E., Willemze R., Falkenburg J. H. Regulatory effects of T cell lymphokines on cytokine gene expression in monocytes. Lymphokine Cytokine Res. 1993 Dec;12(6):457–464. [PubMed] [Google Scholar]
- Conti P., Feliciani C., Barbacane R. C., Panara M. R., Reale M., Placido F. C., Sauder D. N., Dempsey R. A., Amerio P. Inhibition of interleukin-1 beta mRNA expression and interleukin-1 alpha and beta secretion by a specific human recombinant interleukin-1 receptor antagonist in human peripheral blood mononuclear cells. Immunology. 1992 Oct;77(2):245–250. [PMC free article] [PubMed] [Google Scholar]
- Cork M. J., Tarlow J. K., Blakemore A. I., Mee J. B., Crane A. M., Stierle C., Bleehen S. S., Duff G. W. Psoriasis and interleukin-1. A translation. J R Coll Physicians Lond. 1993 Oct;27(4):366–366. [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., Franic G. M., Rathjen D. A., Brooks P. M. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha) and IL-6 on the production of immunoreactive IL-1 and TNF-alpha by human monocytes. Clin Exp Immunol. 1991 Jul;85(1):143–150. doi: 10.1111/j.1365-2249.1991.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., Franic G. M., Rathjen D. A., Laurent R. M., Brooks P. M. Circulating cytokine levels in patients with rheumatoid arthritis: results of a double blind trial with sulphasalazine. Ann Rheum Dis. 1992 Aug;51(8):946–950. doi: 10.1136/ard.51.8.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., Kulesz A. J., Kelly D. E., Nelson D. S., Brooks P. M. The effect of gold treatment on monocyte interleukin-1 production in rheumatoid arthritis. A prospective study. Rheumatol Int. 1990;10(4):153–158. doi: 10.1007/BF02274840. [DOI] [PubMed] [Google Scholar]
- Danis V. A., Kulesz A. J., Nelson D. S., Brooks P. M. Cytokine regulation of human monocyte interleukin-1 (IL-1) production in vitro. Enhancement of IL-1 production by interferon (IFN) gamma, tumour necrosis factor-alpha, IL-2 and IL-1, and inhibition by IFN-alpha. Clin Exp Immunol. 1990 Jun;80(3):435–443. doi: 10.1111/j.1365-2249.1990.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., Kulesz A. J., Nelson D. S., Brooks P. M. The effect of gold sodium thiomalate and auranofin on lipopolysaccharide-induced interleukin-1 production by blood monocytes in vitro: variation in healthy subjects and patients with arthritis. Clin Exp Immunol. 1990 Mar;79(3):335–340. doi: 10.1111/j.1365-2249.1990.tb08092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., March L. M., Nelson D. S., Brooks P. M. Interleukin-1 secretion by peripheral blood monocytes and synovial macrophages from patients with rheumatoid arthritis. J Rheumatol. 1987 Feb;14(1):33–39. [PubMed] [Google Scholar]
- Endres S., Cannon J. G., Ghorbani R., Dempsey R. A., Sisson S. D., Lonnemann G., Van der Meer J. W., Wolff S. M., Dinarello C. A. In vitro production of IL 1 beta, IL 1 alpha, TNF and IL2 in healthy subjects: distribution, effect of cyclooxygenase inhibition and evidence of independent gene regulation. Eur J Immunol. 1989 Dec;19(12):2327–2333. doi: 10.1002/eji.1830191222. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Lonnemann G., van der Meer J. W., Dinarello C. A. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988 Dec;49(3):424–438. doi: 10.1016/0090-1229(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Fenton M. J., Buras J. A., Donnelly R. P. IL-4 reciprocally regulates IL-1 and IL-1 receptor antagonist expression in human monocytes. J Immunol. 1992 Aug 15;149(4):1283–1288. [PubMed] [Google Scholar]
- Fiehn C., Wermann M., Pezzutto A., Hüfner M., Heilig B. GM-CSF-Plasmakonzentrationen bei rheumatoider Arthritis, systemischem Lupus erythematodes und Spondylarthropathie. Z Rheumatol. 1992 May-Jun;51(3):121–126. [PubMed] [Google Scholar]
- Field M., Clinton L. Expression of GM-CSF receptor in rheumatoid arthritis. Lancet. 1993 Nov 13;342(8881):1244–1244. doi: 10.1016/0140-6736(93)92229-m. [DOI] [PubMed] [Google Scholar]
- Granowitz E. V., Vannier E., Poutsiaka D. D., Dinarello C. A. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood. 1992 May 1;79(9):2364–2369. [PubMed] [Google Scholar]
- Hallek M., Lepisto E. M., Slattery K. E., Griffin J. D., Ernst T. J. Interferon-gamma increases the expression of the gene encoding the beta subunit of the granulocyte-macrophage colony-stimulating factor receptor. Blood. 1992 Oct 1;80(7):1736–1742. [PubMed] [Google Scholar]
- Jacob C. O., Fronek Z., Lewis G. D., Koo M., Hansen J. A., McDevitt H. O. Heritable major histocompatibility complex class II-associated differences in production of tumor necrosis factor alpha: relevance to genetic predisposition to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1233–1237. doi: 10.1073/pnas.87.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Mykytyn K., Tashman N. DNA polymorphism in cytokine genes based on length variation in simple-sequence tandem repeats. Immunogenetics. 1993;38(4):251–257. doi: 10.1007/BF00188801. [DOI] [PubMed] [Google Scholar]
- Kuhl D., de la Fuente J., Chaturvedi M., Parimoo S., Ryals J., Meyer F., Weissmann C. Reversible silencing of enhancers by sequences derived from the human IFN-alpha promoter. Cell. 1987 Sep 25;50(7):1057–1069. doi: 10.1016/0092-8674(87)90172-3. [DOI] [PubMed] [Google Scholar]
- Mansfield J. C., Holden H., Tarlow J. K., Di Giovine F. S., McDowell T. L., Wilson A. G., Holdsworth C. D., Duff G. W. Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology. 1994 Mar;106(3):637–642. doi: 10.1016/0016-5085(94)90696-3. [DOI] [PubMed] [Google Scholar]
- Muzio M., Re F., Sironi M., Polentarutti N., Minty A., Caput D., Ferrara P., Mantovani A., Colotta F. Interleukin-13 induces the production of interleukin-1 receptor antagonist (IL-1ra) and the expression of the mRNA for the intracellular (keratinocyte) form of IL-1ra in human myelomonocytic cells. Blood. 1994 Apr 1;83(7):1738–1743. [PubMed] [Google Scholar]
- Mølvig J., Baek L., Christensen P., Manogue K. R., Vlassara H., Platz P., Nielsen L. S., Svejgaard A., Nerup J. Endotoxin-stimulated human monocyte secretion of interleukin 1, tumour necrosis factor alpha, and prostaglandin E2 shows stable interindividual differences. Scand J Immunol. 1988 Jun;27(6):705–716. doi: 10.1111/j.1365-3083.1988.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Poutsiaka D. D., Mengozzi M., Vannier E., Sinha B., Dinarello C. A. Cross-linking of the beta-glucan receptor on human monocytes results in interleukin-1 receptor antagonist but not interleukin-1 production. Blood. 1993 Dec 15;82(12):3695–3700. [PubMed] [Google Scholar]
- Raj N. B., Cheung S. C., Rosztoczy I., Pitha P. M. Mouse genotype affects inducible expression of cytokine genes. J Immunol. 1992 Mar 15;148(6):1934–1940. [PubMed] [Google Scholar]
- Santamaria P., Gehrz R. C., Bryan M. K., Barbosa J. J. Involvement of class II MHC molecules in the LPS-induction of IL-1/TNF secretions by human monocytes. Quantitative differences at the polymorphic level. J Immunol. 1989 Aug 1;143(3):913–922. [PubMed] [Google Scholar]
- Tarlow J. K., Blakemore A. I., Lennard A., Solari R., Hughes H. N., Steinkasserer A., Duff G. W. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993 May;91(4):403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- Tilg H., Trehu E., Atkins M. B., Dinarello C. A., Mier J. W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994 Jan 1;83(1):113–118. [PubMed] [Google Scholar]
- Tilg H., Vannier E., Vachino G., Dinarello C. A., Mier J. W. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993 Nov 1;178(5):1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Costa G. L., Corcoran M., Wahl L. M., Berger A. E. Transforming growth factor-beta mediates IL-1-dependent induction of IL-1 receptor antagonist. J Immunol. 1993 Apr 15;150(8 Pt 1):3553–3560. [PubMed] [Google Scholar]
- Wanidworanun C., Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993 Dec 15;151(12):6853–6861. [PubMed] [Google Scholar]
- Wilson A. G., di Giovine F. S., Blakemore A. I., Duff G. W. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992 Aug;1(5):353–353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. D., Firestein G. S., Taetle R., Kaushansky K., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989 Mar;83(3):876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C. G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J. E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993 Dec 1;151(11):6370–6381. [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C. G., de Vries J. E. Effects of interleukin 4 on monocyte functions: comparison to interleukin 13. Res Immunol. 1993 Oct;144(8):629–633. doi: 10.1016/s0923-2494(05)80016-1. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Takhsh E., Blakemore A. I., Duff G. W. Single base polymorphism at -511 in the human interleukin-1 beta gene (IL1 beta). Hum Mol Genet. 1992 Sep;1(6):450–450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]


