Abstract
We have generated mice deficient in the expression of the lymphocyte cell surface antigen CD48 (Blast-1, BCM1, sgp-60) by gene targeting in embryonic stem cells. Mice homozygous for the CD48 mutation (CD48−/− mice) are severely impaired in CD4+ T cell activation. Proliferative responses to mitogens, anti-CD3 mAb, and alloantigen are all reduced. Experiments in which T cells and antigen-presenting cells from either wild-type or CD48−/− mice were cocultured reveal that CD48 is important on both T cells and antigen-presenting cells. The most dramatic impairment was observed in experiments in which highly purified T cells were stimulated through the T cell receptor in the presence of the phorbol ester, phorbol 12-myristate 13-acetate. The results of these experiments raise the possibility that CD48 plays a role in signaling through the T cell receptor.
Besides the T cell receptor, other cell surface molecules participate in the interaction of T cells with antigen-presenting cells (APCs). One of these molecules is CD2, a 55-to 60-kDa protein expressed on the surface of T cells and natural killer cells; in mice, CD2 also is found on B cells (1). There are two known ligands for CD2. In mice (2) and rats (3), CD2 interacts with CD48 (Blast-1, BCM1, sgp-60). CD48 is a glycosylphosphatidylinositol-anchored, 45- to 50-kDa molecule whose expression is tightly regulated and restricted to lymphocytes, macrophages, and dendritic cells (2, 4, 5). CD48 is expressed in humans; however, human CD48 does not bind CD2 with high affinity. CD58 (LFA-3), a broadly expressed protein of 55- to 70-kDa, is the major ligand for CD2 in humans (6–8). CD58 is structurally and phylogenetically related to CD48 (9). It is currently unknown whether there is a murine homologue of CD58 (2).
Although the dual capacity of CD2, to promote T cell-APC adhesion and to transduce T cell activation signals, is well established (10, 11), the physiologic functions of CD2 and of its ligands have remained uncertain. Mice carrying a targeted mutation in the Cd2 gene are phenotypically almost comparable to wild-type mice (12, 13). However, numerous studies using blocking mAbs suggest that CD2 and its ligands participate in immune responses. Antibodies against murine CD2 can be potent suppressants of cell-mediated immunity and can prolong allograft and xenograft survival (14–16). These mAbs also inhibit T cell-dependent B cell activation, indicating that CD2 and its ligands may play a role in humoral immunity (17). Anti-CD48 mAbs also can inhibit immune responses. They can inhibit the proliferation of T cells in vitro (4) and can suppress cell-mediated immunity in vivo, blocking hapten-induced contact sensitivity and the generation of cytotoxic T lymphocytes (18). Anti-CD48 mAbs also can prolong allograft survival (19). To determine the function of CD48 more definitively, we have generated, by gene targeting, mice deficient in CD48 expression (CD48−/− mice). The results of our experiments demonstrate that CD4+ T cells from CD48−/− mice are defective in activation.
MATERIALS AND METHODS
Animal Experimentation.
All animal experimentation was carried out in accordance with institutional and governmental guidelines.
Homologous Recombination Construct and Targeting of Embryonic Stem (ES) Cells.
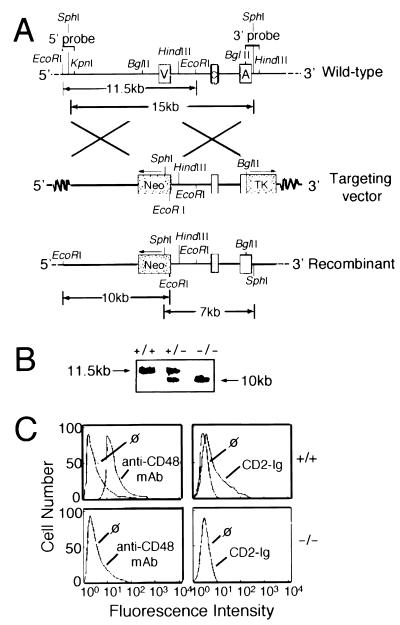
We initially isolated CD48 genomic clones from a 129 genomic λ DASH II library, using the CD48 cDNA insert that previously had been isolated (20). Sequencing of overlapping genomic clones revealed four distinct exons that correspond to the postulated leader peptide, IgV-like domain, IgC-like domain, and anchor region (J.G.-C., G.J.F., and H.R., unpublished work). Clone λ-CD48I hybridized with the oligonucleotides corresponding to the predicted V-like domain, C-like domain, and anchor region. Digestion with SalI released two inserted fragments that were subcloned into the SalI site of pBluescript II SK(+) plasmid (Stratagene) for subsequent manipulation. Clone pBS-CD48I9 contains the V domain and the 5′ portion of the C domain whereas clone pBS-CD48I3 contains the 3′ portion of the C domain and the anchor region. By using the isolated clones pBS-CD48I9 and pBS-CD48I3, a CD48 targeting vector was constructed by replacing the V region with a neomycin gene driven by the pGK promoter and linked to the pGK poly(A) segment. A thymidine kinase gene was incorporated at the 3′ end of the targeting vector. Homologous recombination constructs were introduced into the 129SvJ ES cell line J1 by electroporation, and ES cells were subjected to G418 and FIAU selection (21). G418R and FIAUR colonies were analyzed by Southern blotting, using 5′ and 3′ external probes. Hybridization of EcoRI-digested DNA with the 5′ end EcoRI-KpnI external probe was expected to show a 11.5-kilobase fragment from the wild-type locus and a 10-kilobase fragment from the targeted locus whereas hybridization of SphI-digested DNA with a 3′ BglII-HindIII probe would predict a 15-kilobase fragment from the wild-type locus and a 7-kilobase fragment from the targeted locus. Five clones were identified that carried the Cd48 mutation. These clones were microinjected into blastocysts from C57BL/6 mice.
Chemicals.
All chemicals were obtained from Sigma, unless otherwise specified.
Immunofluorescence and Flow Cytometry.
Thymus and spleen cells were analyzed by immunofluorescence and flow cytometry as described (4). For this purpose, we used a panel of fluorescein isothiocyanate and phycoerythrin (PE)-conjugated antibodies that were purchased in purified form from PharMingen. For staining with CD2-Ig (2), cells were incubated with purified fusion protein, followed by incubation with biotin-conjugated donkey-anti-human IgG (Kirkegaard & Perry Laboratories) and, finally, by incubation with PE-avidin (Sigma). Control stainings included stainings with developing reagents (biotin-conjugated donkey-anti human IgG followed by PE-avidin) only or staining with isotype controls. For the sake of clarity, the staining profiles of these control stainings are omitted in several of the figures.
Cell Preparations and Cultures.
Single cell suspensions were prepared from spleen and thymus and were depleted of erythrocytes as described (4). The cells were washed and resuspended in complete culture medium (RPCCM) consisting of RPMI medium 1640 (GIBCO/BRL) supplemented as described (4). Where indicated in the figure legends, CD4+ T cells were purified as described by negative depletion with anti-major histocompatibility complex class II and anti-CD8 mAbs (22). This procedure typically results in >98% pure CD4+ cells as evidenced by fluorescence-activated cell sorter analysis (ref. 22 and data not shown). Microcultures (200 μl) were set up in triplicate as described (4); the precise culture constituents and incubation periods are described in the respective figure legends. T cell proliferation and interleukin 2 production were assayed as described (4).
RESULTS AND DISCUSSION
Generation of CD48-Deficient Mice.
We initially isolated CD48 genomic clones from a 129 genomic λ DASH II library, using the CD48 cDNA insert that previously had been isolated (20). A schematic map of the murine Cd48 gene is illustrated in Fig. 1A; the precise organization of the locus is described in detail elsewhere (J.G.-C., G.J.F., and H.R., unpublished work). To inactivate the expression of CD48, the IgV-like domain was deleted completely and was replaced with a neomycin cassette in antisense orientation by homologous recombination in ES cells. The IgV-like domain of CD48 was chosen for gene targeting because it forms the binding site for CD2. To select against random insertional events, a thymidine kinase gene was incorporated at the 3′ end of the targeting vector (Fig. 1A). Among 240 ES clones analyzed, we identified 5 that carried the CD48 mutation. For each of these clones, hybridization with an internal neomycin probe yielded a single band, indicating that the clones were the result of single integration events. All five clones yielded chimeric mice that subsequently transmitted the mutation through the germline. Germline-transmitting chimeras were back-crossed onto C57BL/6 mice. Heterozygotes were intercrossed, and the offspring were typed by Southern blot analysis using 5′ and 3′ probes (Fig. 1B and data not shown). Homozygous mutant mice were present at the expected Mendelian frequency (data not shown). Two of the founder lines were selected randomly for further study and were back-crossed for five generations. Staining and functional experiments have been carried out with CD48−/− mice derived from either of these founders; experiments with both lines have yielded results that are indistinguishable.
Figure 1.
Targeted disruption of the Cd48 gene by homologous recombination. (A) Experimental strategy. The upper panel illustrates the organization of the Cd48 gene. Indicated are the relative positions of the IgV-like domain (V), the IgC-like domain (C), and the membrane-anchoring domain (A). The middle panel illustrates the targeting vector; the relative positions of the neomycin (neo) and thymidine kinase (TK) cassettes are indicated. The lower panel illustrates the resulting recombinant. (B) Southern blot analysis of CD48-deficient mice. Genomic DNAs prepared from tail biopsies were digested with EcoRI and were subjected to hybridization with the 5′ end EcoRI-KpnI probe. Homozygous offspring were present at the expected Mendelian frequency. +/+, wild-type; ±, heterozygous mutant; −/−, homozygous mutant. (C) Expression of CD2 ligands in CD48-deficient mice. Thymocytes from wild-type (Upper) or CD48−/− (Lower) mice were analyzed by immunofluorescence and flow cytometry. To assess CD48 expression, cells were stained with PE-conjugated anti-CD48 mAb (Left). To assess expression of CD2 ligands other than CD2, cells were stained with biotinylated CD2-Ig followed by incubation with biotin-conjugated donkey-anti-human IgG and, finally, by incubation with PE-avidin (Right). All samples were analyzed on a FACScan (Becton Dickinson). Five-thousand cells were analyzed per sample. The symbol “ø” indicates control staining.
Cells from CD48−/− Mice Lack Surface Expression of the CD48 Protein and Reactivity with CD2-Ig Fusion Protein.
To confirm the absence of CD48 protein expression, we analyzed thymocytes and splenocytes from CD48−/− mice and wild-type littermate controls. A representative experiment is shown in Fig. 1C. Although thymocytes from CD48+/+ mice were readily stained with anti-CD48 mAb (Fig. 1C Upper Left), no expression was detected on CD48−/− thymocytes (Fig. 1C Lower Left). These data indicate that the expression of CD48 was extinguished successfully in CD48−/− mice.
Several years ago, based on chromosomal mapping studies, Williams, Seldin, and their colleagues suggested that mice do not express a homologue of the human CD58 (LFA-3) antigen (9). To address this point, we analyzed thymocytes and splenocytes from CD48−/− mice with CD2-Ig, a chimeric fusion protein comprising the extracellular domain of murine CD2 and the hinge-CH2-CH3 domains of the human IgG1 heavy chain. This reagent binds to CD48 on wild-type lymphoid cells as well as to CD48-transfected Chinese hamster ovary cells (2). The results of flow cytometric studies indicate that lymphoid cells from CD48+/+ (Fig. 1C Upper Right) but not CD48−/− mice (Fig. 1C Lower Right) bind CD2-Ig. These findings are consistent with the notion (9) that mice do not express a homologue of CD58.
Lymphoid Development in CD48−/− Mice.
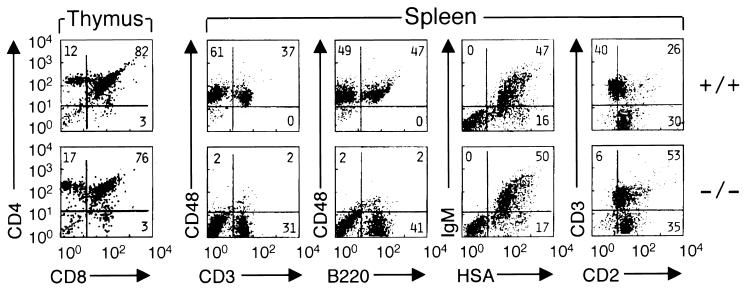
CD48−/− mice develop normally and are healthy, at least up to 3 months of age. We have not detected gross abnormalities in body weight or organ size. Lymphoid development in CD48-deficient mice appears grossly normal, as judged from the total number of lymphocytes in thymus spleen and lymph node. Similarly, the expression of CD3, CD28, Thy-1, B220, IgM, B7–1 (CD80), B7–2 (CD86) and heat-stable antigen on thymocytes and splenocytes from CD48−/− mice was indistinguishable from wild-type mice (Fig. 2 and data not shown).
Figure 2.
Lymphoid development in CD48-deficient mice. Thymocytes and splenocytes were purified from CD48+/+ mice (upper row) and from CD48−/− mice (lower row) and were analyzed by dual color immunofluorescence and flow cytometry. All samples were analyzed on a FACScan after compensation. Five-thousand cells were analyzed per sample. Antigens displayed on the y axes were stained with PE-conjugated mAbs whereas antigens on the x axes were stained with fluorescein isothiocyanate-conjugated mAbs.
Two developmental alterations are observed in CD48−/− mice. First, although all of the four major subsets are present in the thymus, we have consistently (n = 7) observed in CD48−/− mice a small increase in the fraction of CD4+ CD8− thymocytes (Fig. 2). Consistent with these observations, similar results were obtained when the number of CD4+ spleen cells was analyzed (data not shown). The significance of these alterations, if any, remains to be determined. Secondly, the expression of the CD2 antigen is up-regulated in thymocytes and spleen cells of CD48−/− mice (Fig. 2 and data not shown; n = 10). The most probable explanation for this result is that the expression of CD2 is regulated by its ligand CD48 and that the extinction of CD48 leads to a dysregulation of CD2 expression. A similar result has been observed in the case of major histocompatibility complex Class II-deficient mice in which the expression of CD4 is up-regulated (23).
CD48−/− Splenocytes Display Reduced Responsiveness to T Cell Mitogens and Anti-CD3 mAb.
Previous studies have shown that certain anti-CD48 mAbs can inhibit the proliferation of CD4+ T lymphocytes normally induced by mitogenic lectins or by anti-CD3 mAb. In most of the studies (2, 18), the most dramatic effects were observed on responses stimulated by phytohemagglutinin (PHA) whereas Con A and anti-CD3 mAb-induced responses were less affected.
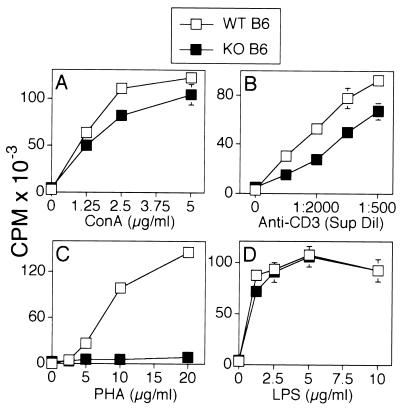
To determine the activation potential of T cells from CD48−/− mice, we initially studied the proliferative responses of unfractionated spleen cells to lectins and anti-CD3 mAb. Splenocytes from CD48+/+ or CD48−/− mice were cultured over a timecourse of 12–96 hr, with different concentrations of Con A, anti-CD3 mAb, or PHA. The time course and the concentration of stimuli were varied to discern kinetic or threshold differences. A representative experiment is shown in Fig. 3. The proliferative responses of CD48−/− splenocytes to Con A and anti-CD3 mAb were reduced, although the differences were relatively small (range is ≈20–50%; Fig. 3 A and B). The most dramatic effect of the CD48 mutation was seen when splenocytes were stimulated with PHA. Proliferative responses of CD48−/− splenocytes to this lectin were essentially abrogated (Fig. 3C). Similar effects were observed when the production of interleukin 2 was analyzed instead of proliferative responses (data not shown).
Figure 3.
Proliferation of spleen cells from CD48-deficient mice in response to stimulation with lectins or anti-CD3 mAb. (A) Splenocytes (2 × 105) from wild-type (WT, □) and CD48−/− mice (KO, ■) were stimulated in triplicate with Con A (A), anti-CD3 mAb 145–2C11 (B), PHA (C), or lipopolysaccharide (LPS; D) at the concentrations indicated on the x axes. All cultures were pulsed with 1 μCi (37 kBq) of [3H]thymidine per well for the last 6–8 hr of the 48-hr incubation period to assay for T cell proliferation. Results are representative of nine independent experiments.
Although the CD48 mutation affected the activation of splenocytes by T cell stimuli, no defect was observed when splenocytes were activated with the B cell mitogen lipopolysaccharide. Over a time course of 12–96 hr, no difference was observed in the capacity of CD48+/+ and CD48−/− splenocytes to proliferate in response to stimulation with lipopolysaccharide (Fig. 3D). This finding raises the possibility that T cell-independent activation of B cells may not be affected by the CD48 mutation.
Diminished Alloresponses of CD48−/− Mice: CD48 Is Functional on Both T cells and Stimulator Cells.
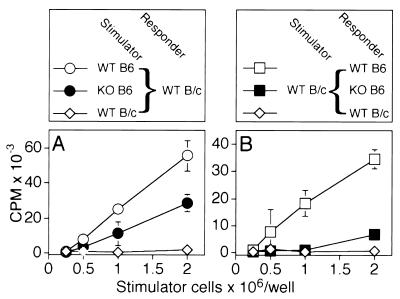
To examine the function of CD48 in a more physiologic situation, we analyzed allo-mixed lymphocyte reactions. This experimental approach also allowed us to assess separately the functional significance of CD48 on T cells and on stimulator cells. BALB/c mice express H-2d class II antigens whereas C57BL/6 mice express H-2b. CD4+ T cells from one of the strains, therefore, proliferate in response to class II-bearing cells of the other strain. To assess the role of CD48 on stimulator cells, highly purified CD4+ T cells from BALB/c mice were stimulated with irradiated splenocytes from either C57BL/6 wild-type or C57BL/6 CD48−/− mice. In contrast, to assess the function of CD48 on CD4+ T cells, CD4+ T cells were purified from either C57BL/6 wild-type or C57BL/6 CD48−/− mice, and each population was stimulated with the identical population of irradiated BALB/c wild-type splenocytes. Cells were cocultured for 4, 5, 6, or 7 days to determine differences in the kinetics of responses, although the proliferative responses peaked at day 5 or day 6 irrespective of whether cells from either wild-type or CD48−/− mice were analyzed.
A representative experiment is shown in Fig. 4. When compared with C57BL/6 wild-type mice, stimulator cells from C57BL/6 CD48−/− mice had a reduced capacity to stimulate the proliferation of BALB/c T cells, although the effect of the CD48 mutation was incomplete (Fig. 4A). In four independent experiments, responses to CD48-deficient stimulator cells were 53.2% (range was 32.5–75.7%; 2 × 106 stimulator cells) of those induced by wild-type stimulator cells. In the reverse experiment, the proliferative response of C57BL/6 CD48−/− T cells to BALB/c stimulator cells was reduced greatly (Fig. 4B). In four independent experiments, responses of CD48-deficient CD4+ T cells were 19.8% (range was 11.5–25.9%; 2 × 106 stimulator cells) of those of wild-type T cells. Similar results were obtained when interleukin 2 production was analyzed instead of T cell proliferation (data not shown). Taken together, these experiments indicate that CD48 is functional on both T cells and stimulator cells during alloresponses.
Figure 4.
Role of CD48 in allo-mixed lymphocyte reactions. In A, cultures were constructed by using 2 × 105 T cells from BALB/c wild-type mice (WT B/c) as responders. Irradiated (3,000 rads) spleen cells from C57BL/6 wild-type mice (WT B6, ○), CD48−/− C57BL/6 mice (KO B6, •), or BALB/c wild-type mice (WT B/c, ⋄) were added as stimulators in the numbers indicated on the x axis. In B, irradiated (3,000 rads) spleen cells from BALB/c wild-type mice (WT B/c) were used as stimulators as indicated on the x axis. Cultures were set up by using T cells from C57BL/6 wild-type mice (WT B6, □), CD48−/− C57BL/6 mice (KO B6, ■), or BALB/c wild-type mice (B/c WT, ⋄) were added as responders. All cultures were set up in triplicate and were pulsed with 1 μCi (37 kBq) of [3H]thymidine per well for the last 18 hr of the 5-day incubation period to assay for T cell proliferation. Qualitatively similar results were obtained when T cell proliferation was measured on days 4, 6, and 7 after initiation of the culture (data not shown). Results are representative of five independent experiments
Proliferative Responses of Highly Purified CD4+ CD48-Deficient T Cells to T Cell Receptor Stimulation in the Presence of Phorbol 12-Myristate 13-Acetate (PMA) Are Abrogated.
The experiments analyzing allo-mixed lymphocyte reactions indicated that CD48 on T cells is important for the response. To further examine the function of CD48 on T cells, we exploited the fact that highly purified CD4+ T cells can proliferate in response to anti-CD3 mAb or lectins when the phorbol ester PMA is added to the culture medium as a costimulatory signal. It had been shown that anti-CD48 mAb can inhibit the activation of highly purified CD4+ T cells induced by anti-CD3 mAb and PMA (4).
CD4+ T lymphocytes were purified from wild-type or CD48−/− mice as described and were stimulated with either anti-CD3 mAb and PMA or with Con A and PMA. As shown in Fig. 5 A and B, respectively, the proliferative response of CD48-deficient T cells to these stimuli was essentially abrogated. In contrast, the proliferative response of CD48−/− and CD48+/+ T cells to a mitogenic combination of calcium ionophore and PMA was identical (Fig. 5C). Therefore, CD48-deficient T cells can proliferate when cell surface receptors are circumvented. It is noteworthy that the data of these experiments with CD48 knockout mice are entirely consistent with prior studies analyzing the inhibitory effects of anti-CD48 mAb on the activation of wild-type T cells (4).
Figure 5.
Proliferation of highly purified CD4+ T cells from CD48-deficient mice in response to T cell receptor stimulation in the presence of PMA. Purified T cells (>98% CD4+) were prepared from wild-type (WT, □) and CD48-deficient mice (KO, ■) as described (4) and were incubated at a density of 106 cells/ml in the presence of PMA (10 ng/ml). Con A (A), anti-CD3 mAb 145–2C11 (B), and ionomycin (C) were added at the concentrations indicated on the x axes. All cultures were set up in triplicate and were pulsed with 1 μCi (37 kBq) of [3H]thymidine per well for the last 6–8 hr of the 48-hr incubation period to assay for T cell proliferation. Results are representative of eight independent experiments
Concluding Remarks.
The results of our experiments indicate that CD48 plays an important role in T cell activation through the T cell receptor/CD3 complex. T cells from CD48-deficient mice display reduced proliferation in response to stimulation with lectins, anti-CD3 mAb, and alloantigen. The most dramatic impairment was observed in experiments in which the phorbol ester PMA was used as a costimulatory signal instead of APCs. It is noteworthy that the addition of irradiated APCs to the culture partially reconstituted the T cell proliferative responses (data not shown), consistent with the results shown in Fig. 3. The results of the experiments in which PMA was used as a costimulator instead of APCs raise the possibility that CD48 plays a role in T cell signaling. This possibility will need to be explored further. Similarly, it will be necessary to carry out in vivo studies to delineate the precise role of CD48 in cellular and humoral immunity.
The most interesting implication of our study is that the phenotype of CD48-deficient mice is more dramatic than that reported for the CD2 knockout (12, 13). The most plausible explanation for the different phenotypes of the CD48- and CD2-deficient strains is that there exist other ligands for CD48 besides CD2. Indeed, recent experiments have identified the 2B4 molecule, an activation antigen expressed by natural killer cells and epidermal γδ T cells (24, 25), as a novel CD48 counter-receptor (26). The functional significance of the 2B4-CD48 interaction remains to be determined. However, it is noteworthy that natural killer cells can interact with and stimulate T and B lymphocytes and that these interactions are known to require cell–cell contact (27, 28). We also are currently investigating whether there are other CD48 counter-receptors, besides CD2 and 2B4, that may be expressed by APCs or T cells.
Acknowledgments
This work was supported by grants from the Wellcome Trust (to H.R.) and the National Institutes of Health (to A.H.S.).
ABBREVIATIONS
- APC
antigen-presenting cell
- ES cells
embryonic stem cells
- PHA
phytohemagglutinin
- PMA
phorbol 12-myristate 13-acetate
- PE
phycoerythrin
References
- 1.Davis S J, van der Merwe P A. Immunol Today. 1996;17:177–187. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- 2.Kato K, Koyanagi M, Okada H, Takanashi T, Wong Y-W, Williams A F, Okumura K, Yagita H. J Exp Med. 1992;176:1241–1249. doi: 10.1084/jem.176.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Merwe P A, McPherson D C, Brown M H, Barclay A N, Cyster J G, Williams A F, Davis S J. Eur J Immunol. 1993;23:1373–1377. doi: 10.1002/eji.1830230628. [DOI] [PubMed] [Google Scholar]
- 4.Reiser H. J Immunol. 1990;145:2077–2086. [PubMed] [Google Scholar]
- 5.Thorley-Lawson D A, Schooley R T, Bhan A K, Nadler L M. Cell. 1982;30:415–425. doi: 10.1016/0092-8674(82)90239-2. [DOI] [PubMed] [Google Scholar]
- 6.Selvaraj P, Plunkett M L, Dustin M, Sanders M E, Shaw S, Springer T A. Nature (London) 1987;326:400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- 7.Dustin T A, Sanders M E, Shaw S, Springer T A. J Exp Med. 1987;165:677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arulanandam A R N, Kister A, McGregor M J, Wyss D F, Wagner G, Reinherz E L. J Exp Med. 1994;180:1861–1871. doi: 10.1084/jem.180.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong Y H, Williams A F, Kingsmore S F, Seldin M F. J Exp Med. 1990;171:2115–2130. doi: 10.1084/jem.171.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, Takahashi K, Fukazawa T, Koyanagi M, Yokoyama A, Kato H, Yagita H, Okumura K. J Immunol. 1990;145:3628–3634. [PubMed] [Google Scholar]
- 11.Meuer S C, Hussey R E, Fabbi M, Fox D, Acuto O, Fitzgerald K A, Hodgdon J C, Protentis J P, Schlossman S F, Reinherz E L. Cell. 1984;36:897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- 12.Killeen N, Stuart S G, Littman D R. EMBO J. 1992;11:4329–4336. doi: 10.1002/j.1460-2075.1992.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teh S J, Killeen N, Tarakhovsky A, Littman D R, Teh H S. Blood. 1997;89:1308–1318. [PubMed] [Google Scholar]
- 14.Bromberg J S, Chavin K D, Altevogt P, Kyewski B A, Guckel B, Naji A, Barker C F. Transplantation. 1991;51:219–225. doi: 10.1097/00007890-199101000-00036. [DOI] [PubMed] [Google Scholar]
- 15.Guckel B, Berek C, Lutz M, Altevogt P, Schirrmacher V, Kyewski B A. J Exp Med. 1991;174:957–967. doi: 10.1084/jem.174.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chavin K D, Lau H T, Bromberg J S. Transplantation. 1992;54:286–291. doi: 10.1097/00007890-199208000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Sen J, Bossu P, Burakoff S J, Abbas A K. J Immunol. 1992;148:1037–1042. [PubMed] [Google Scholar]
- 18.Chavin K D, Qin L, Lin J, Woodward J, Baliga P, Kato K, Yagita H, Bromberg J S. Int Immunol. 1994;6:701–709. doi: 10.1093/intimm/6.5.701. [DOI] [PubMed] [Google Scholar]
- 19.Qin L, Chavin K D, Lin J, Yagita H, Bromberg J S. J Exp Med. 1994;179:341–346. doi: 10.1084/jem.179.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrero J G, Freeman G, Lane W, Reiser H. Proc Natl Acad Sci USA. 1993;90:3418–3422. doi: 10.1073/pnas.90.8.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tivol E A, Boriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 22.Scheipers P, Reiser H. Proc Natl Acad Sci USA. 1998;95:10083–10088. doi: 10.1073/pnas.95.17.10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosgrove D, Gray D, Dierich D, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 24.Garni-Wagner B A, Purohit A, Mathew P A, Bennett M, Kumar V. J Immunol. 1993;151:60–70. [PubMed] [Google Scholar]
- 25.Schuhmachers G, Ariizumi K, Mathew P A, Bennett M, Kumar V, Takashima A. Eur J Immunol. 1995;25:1117–1120. doi: 10.1002/eji.1830250440. [DOI] [PubMed] [Google Scholar]
- 26.Latchman Y, McKay P F, Reiser H. J Immunol. 1998;161:5809–5812. [PubMed] [Google Scholar]
- 27.Gray J D, Horwitz D A. J Immunol. 1995;154:5656–5664. [PubMed] [Google Scholar]
- 28.Horwitz D A, Gray J D, Ohtsuka K, Hirokawa M, Takahashi T. Immunol Today. 1997;18:538–542. doi: 10.1016/s0167-5699(97)01149-3. [DOI] [PubMed] [Google Scholar]