Abstract
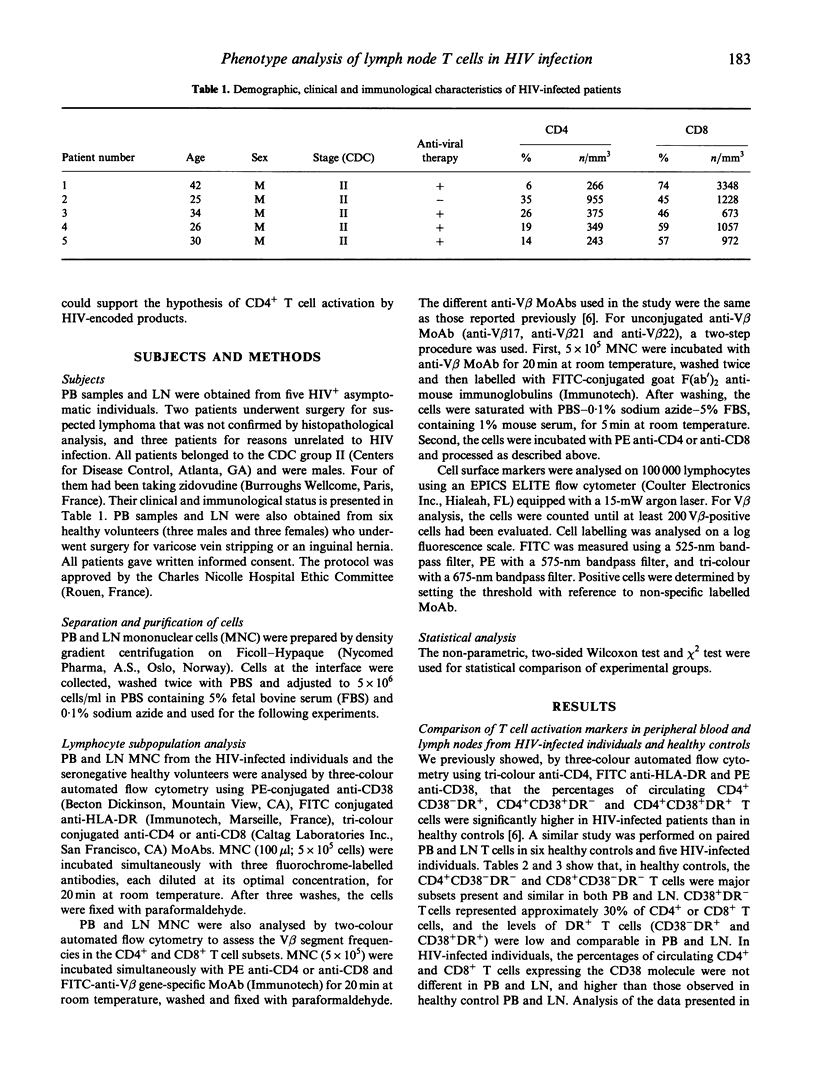
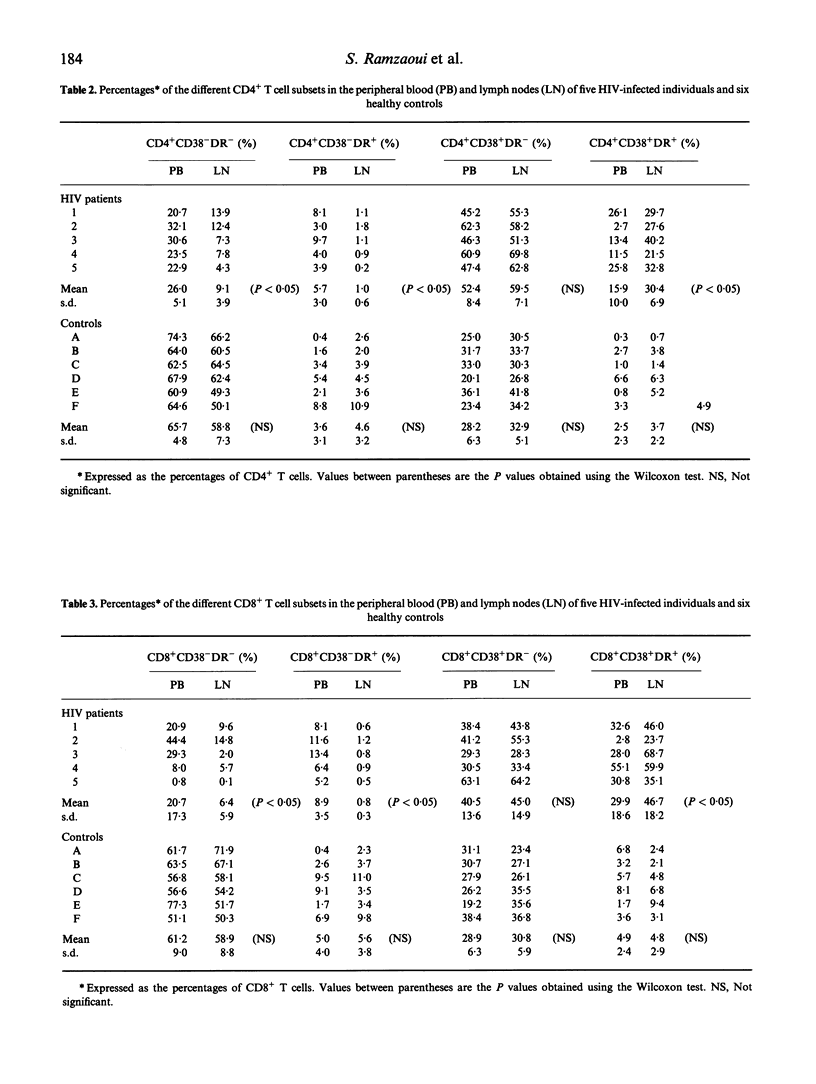
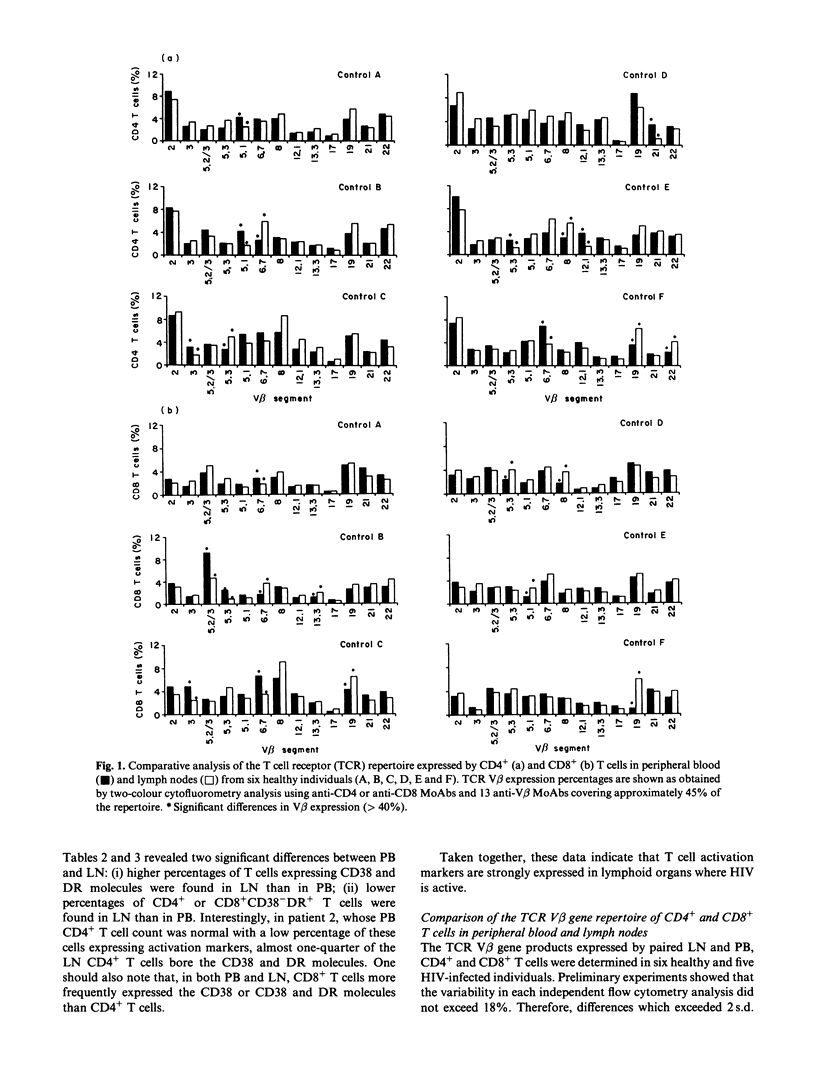
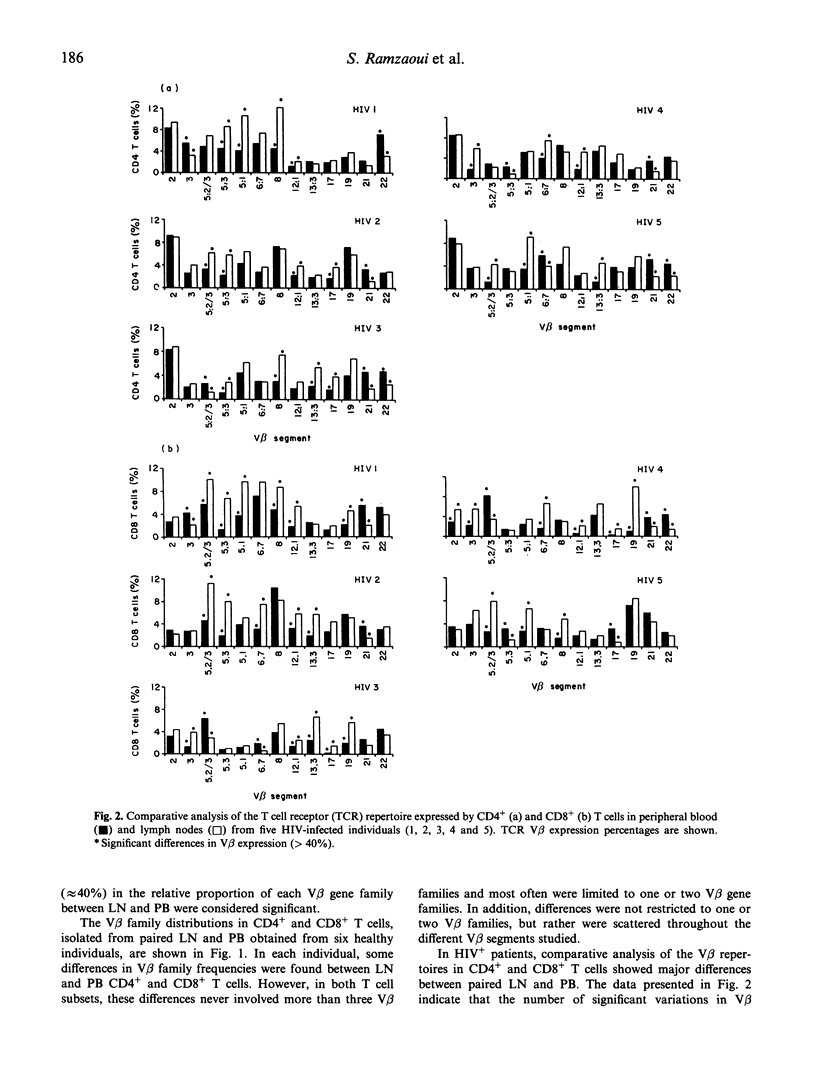
Since lymphoid organs constitute the site of active and progressive HIV disease, analysis of their lymphocytes may provide more accurate information on T cell abnormalities than that obtained from studying peripheral blood lymphocytes. The objective of this study was to compare the expressions of activation markers and T cell receptor (TCR) V beta gene products by CD4+ and CD8+ T cells in lymph nodes (LN) and peripheral blood (PB) from healthy individuals and asymptomatic HIV-infected patients to determine whether anomalies that could be identified at the HIV replication site could support the hypothesis of T cell activation by HIV-encoded antigens or superantigens. CD4+ and CD8+ T cells in paired LN and PB obtained from six healthy controls and five asymptomatic HIV-infected individuals were analysed by flow cytometry, using anti-CD38, anti-HLA-DR and 13 anti-V beta MoAbs that cover, approximately, 45% of the T cell repertoire. Analysis of T cell activation marker expression indicated that the percentages of CD4+ and CD8+ T cells bearing CD38 or CD38 and HLA-DR molecules were higher in patients than in controls and, in patients, higher in LN than in PB. Comparison between the V beta repertoires of CD4+ and CD8+ T cells in LN and PB showed that, in each healthy individual, a limited number of V beta families expressed by CD4+ or CD8+ T cells had different repartition in LN and PB, whereas in each HIV+ patient, more V beta families exhibited different distributions and these differences recurred among certain V beta segments, such as V beta 5.3 and V beta 21 in the CD4+ T cell population and V beta 5.2/5.3, V beta 12 and V beta 21 in the CD8+ T cell population. Taken together, these data argue for a skewed TCR repertoire in HIV infection and sustained activation of T cells by HIV-encoded antigens at the site of HIV replication, and further demonstrate that a high proportion of CD4+ T cells are in an activation state that may, indirectly, participate in their functional abnormalities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher M. S., Sheppard H. W. AIDS as immune system activation. II. The panergic imnesia hypothesis. J Acquir Immune Defic Syndr. 1990;3(2):177–191. [PubMed] [Google Scholar]
- Bansal A. S., Green L. M., Pumphrey R. S., Mandal B. T cells, V genes, and HIV. Lancet. 1992 Jun 27;339(8809):1604–1604. doi: 10.1016/0140-6736(92)91865-6. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Wilson S., Gompels M., Ludlam C., Gazzard B., Coates A. M., Habeshaw J. T-cell receptor variable gene products and early HIV-1 infection. Lancet. 1992 Apr 4;339(8797):824–828. doi: 10.1016/0140-6736(92)90277-a. [DOI] [PubMed] [Google Scholar]
- Imberti L., Sottini A., Bettinardi A., Puoti M., Primi D. Selective depletion in HIV infection of T cells that bear specific T cell receptor V beta sequences. Science. 1991 Nov 8;254(5033):860–862. doi: 10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- Lees O., Ramzaoui S., Gilbert D., Borsa F., Humbert G., Leblanc D., Lagarde M., Tron F. The impaired in vitro production of interleukin-2 in HIV infection is negatively correlated to the number of circulating CD4+DR+ T cells and is reversed by allowing T cells to rest in culture: arguments for in vivo CD4+ T cell activation. Clin Immunol Immunopathol. 1993 Jun;67(3 Pt 1):185–191. doi: 10.1006/clin.1993.1063. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebai N., Pantaleo G., Demarest J. F., Ciurli C., Soudeyns H., Adelsberger J. W., Vaccarezza M., Walker R. E., Sekaly R. P., Fauci A. S. Analysis of the T-cell receptor beta-chain variable-region (V beta) repertoire in monozygotic twins discordant for human immunodeficiency virus: evidence for perturbations of specific V beta segments in CD4+ T cells of the virus-positive twins. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1529–1533. doi: 10.1073/pnas.91.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenesis of HIV infection. FASEB J. 1991 Jul;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- Soudeyns H., Rebai N., Pantaleo G. P., Ciurli C., Boghossian T., Sékaly R. P., Fauci A. S. The T cell receptor V beta repertoire in HIV-1 infection and disease. Semin Immunol. 1993 Jun;5(3):175–185. doi: 10.1006/smim.1993.1021. [DOI] [PubMed] [Google Scholar]


