Abstract
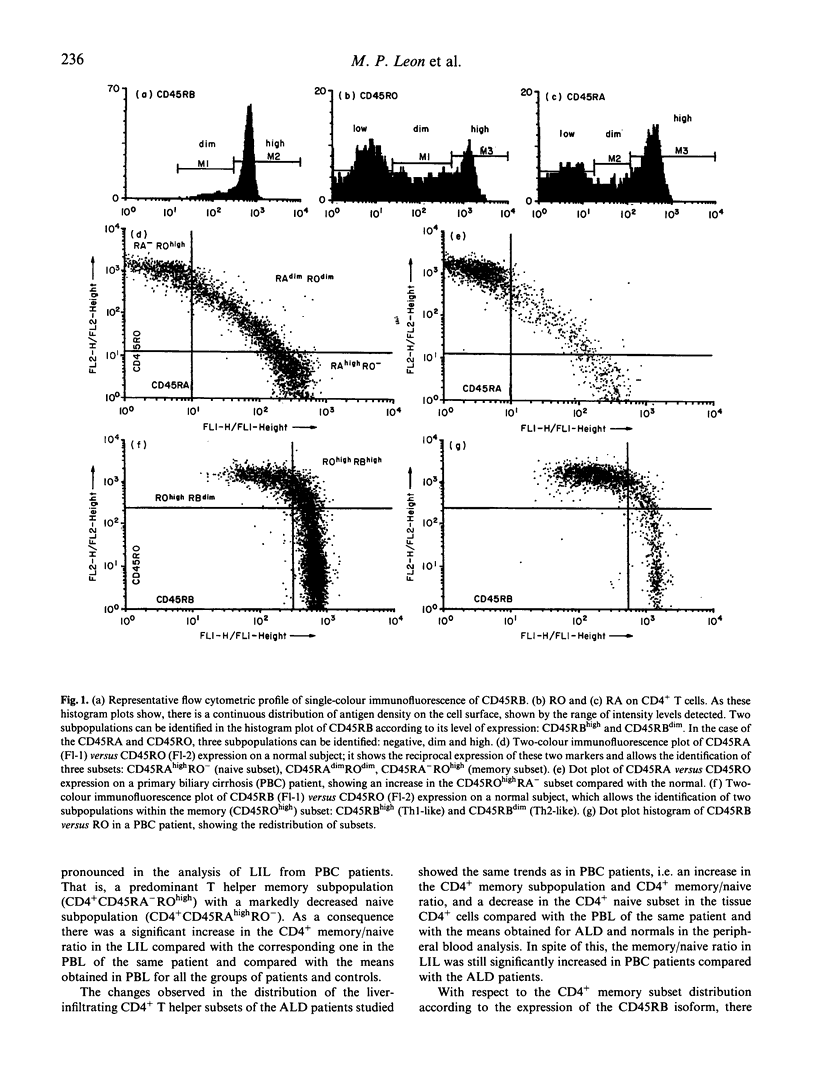
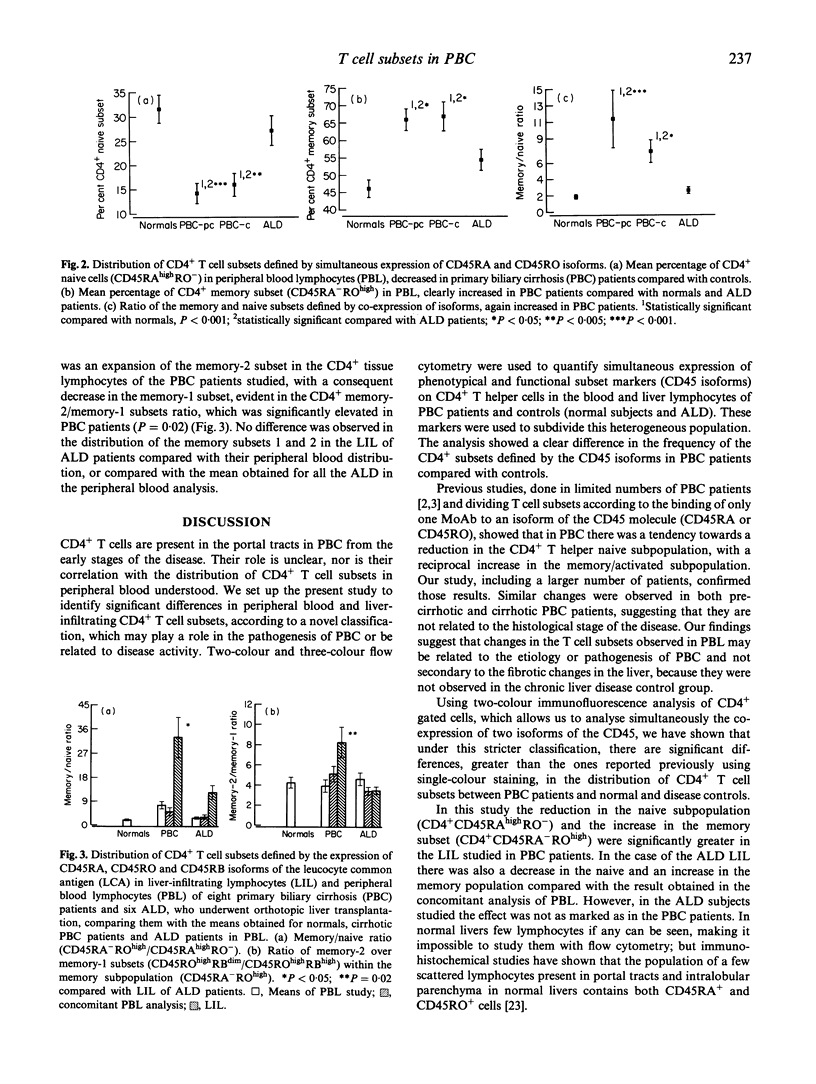
Primary biliary cirrhosis (PBC) is an autoimmune condition characterized by destruction of the intrahepatic bile ducts. Autoreactive CD4+ T cells have been reported both in the peripheral circulation and in the mononuclear cell infiltrate in the affected portal tracts. In this large study we have used two- and three-colour flow cytometry to determine the phenotypes of the CD4+ T cell subsets in the peripheral blood and liver-infiltrating lymphocytes of PBC patients (n = 43), normal controls (n = 19) and patients with alcoholic cirrhosis (n = 15), according to a novel classification based on the simultaneous expression of different isoforms of CD45. In PBC patients the proportion of peripheral blood CD4+ cells possessing the CD45ROhighRA- 'memory' phenotype was significantly increased, and the CD45RO-RAhigh 'naive' population was significantly decreased, compared with the two control groups. No significant differences in peripheral blood CD4+ T cell subsets were seen between patients with pre-cirrhotic and cirrhotic PBC. A similar, but more marked, shift towards the CD45ROhighRA- 'memory' phenotype was seen in the liver-infiltrating CD4+ T cells in PBC patients compared with alcoholic cirrhotics. Cells within the CD4+ memory subpopulation were further subgrouped according to expression of CD45RB, the level of expression of which has been associated with functional differences in the memory subset. In peripheral blood no differences were seen between PBC patients and controls with respect to the proportion of CD45ROhighRBhigh and CD45ROhighRBdim memory subsets. A statistically significant difference in the distribution of these memory subsets, with an increased memory-2/memory-1 ratio was observed in the liver-infiltrating CD4+ T cells of PBC patients compared with those from alcoholic cirrhotic patients. The potential implications of this observation are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992 Feb;4(1):35–41. [PubMed] [Google Scholar]
- Björkland A., Festin R., Mendel-Hartvig I., Nyberg A., Löf L., Tötterman T. H. Blood and liver-infiltrating lymphocytes in primary biliary cirrhosis: increase in activated T and natural killer cells and recruitment of primed memory T cells. Hepatology. 1991 Jun;13(6):1106–1111. doi: 10.1002/hep.1840130617. [DOI] [PubMed] [Google Scholar]
- Fowell D., Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993 Mar 1;177(3):627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell D., McKnight A. J., Powrie F., Dyke R., Mason D. Subsets of CD4+ T cells and their roles in the induction and prevention of autoimmunity. Immunol Rev. 1991 Oct;123:37–64. doi: 10.1111/j.1600-065x.1991.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- Gruber R., Reiter C., Riethmüller G. Triple immunofluorescence flow cytometry, using whole blood, of CD4+ and CD8+ lymphocytes expressing CD45RO and CD45RA. J Immunol Methods. 1993 Aug 9;163(2):173–179. doi: 10.1016/0022-1759(93)90120-v. [DOI] [PubMed] [Google Scholar]
- Horgan K. J., Tanaka Y., Shaw S. Postthymic differentiation of CD4 T lymphocytes: naive versus memory subsets and further specialization among memory cells. Chem Immunol. 1992;54:72–102. [PubMed] [Google Scholar]
- Li X. M., Jeffers L. J., Reddy K. R., de Medina M., Silva M., Villanueva S., Klimas N. G., Esquenazi V., Schiff E. R. Immunophenotyping of lymphocytes in liver tissue of patients with chronic liver diseases by flow cytometry. Hepatology. 1991 Jul;14(1):121–127. doi: 10.1002/hep.1840140120. [DOI] [PubMed] [Google Scholar]
- Luqman M., Johnson P., Trowbridge I., Bottomly K. Differential expression of the alternatively spliced exons of murine CD45 in Th1 and Th2 cell clones. Eur J Immunol. 1991 Jan;21(1):17–22. doi: 10.1002/eji.1830210104. [DOI] [PubMed] [Google Scholar]
- Mackay C. R. Immunological memory. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- Mackay C. R. T-cell memory: the connection between function, phenotype and migration pathways. Immunol Today. 1991 Jun;12(6):189–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- Mason D., Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology. 1990 Aug;70(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- Mason D. Subsets of CD4+ T cells defined by their expression of different isoforms of the leucocyte-common antigen, CD45. Biochem Soc Trans. 1992 Feb;20(1):188–190. doi: 10.1042/bst0200188. [DOI] [PubMed] [Google Scholar]
- Matthews N., Emery P., Pilling D., Akbar A., Salmon M. Subpopulations of primed T helper cells in rheumatoid arthritis. Arthritis Rheum. 1993 May;36(5):603–607. doi: 10.1002/art.1780360505. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., Pino M., Ofosu-Appiah W., Grob D. Phenotypic and functional characterization of T cells from patients with myasthenia gravis. J Clin Invest. 1990 Dec;86(6):2099–2108. doi: 10.1172/JCI114948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Romain P. L., Fox D. A., Anderson P., DiMaggio M., Levine H., Schlossman S. F. Abnormalities in CD4+ T-lymphocyte subsets in inflammatory rheumatic diseases. Am J Med. 1988 May;84(5):817–825. doi: 10.1016/0002-9343(88)90058-7. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K., Braddy S., Liggitt D., Watson J. D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993 Jul 1;178(1):237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Wolf H., Göttlicher J., Eibl M. M. Helper-inducer and suppressor-inducer lymphocyte subsets in alcoholic cirrhosis. Scand J Gastroenterol. 1991 Mar;26(3):295–301. doi: 10.3109/00365529109025045. [DOI] [PubMed] [Google Scholar]
- Richards S. J., Jones R. A., Roberts B. E., Patel D., Scott C. S. Relationships between 2H4 (CD45RA) and UCHL1 (CD45RO) expression by normal blood CD4+CD8-, CD4-CD8+, CD4-CD8dim+, CD3+CD4-CD8- and CD3-CD4-CD8- lymphocytes. Clin Exp Immunol. 1990 Jul;81(1):149–155. doi: 10.1111/j.1365-2249.1990.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. T., Miller N., Alexander D. R. CD3 antigen-mediated calcium signals and protein kinase C activation are higher in CD45R0+ than in CD45RA+ human T lymphocyte subsets. Eur J Immunol. 1993 Jan;23(1):61–68. doi: 10.1002/eji.1830230111. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Alterations in T cell subsets in multiple sclerosis and other autoimmune diseases. Lancet. 1988 Oct 29;2(8618):1021–1021. doi: 10.1016/s0140-6736(88)90773-8. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Thomas R., McIlraith M., Davis L. S., Lipsky P. E. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992 Dec;35(12):1455–1465. doi: 10.1002/art.1780351209. [DOI] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Memory T cells represent the predominant lymphocyte subset in acute and chronic liver inflammation. Hepatology. 1991 May;13(5):826–829. [PubMed] [Google Scholar]
- Yeaman S. J., Fussey S. P., Danner D. J., James O. F., Mutimer D. J., Bassendine M. F. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988 May 14;1(8594):1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]


