Abstract
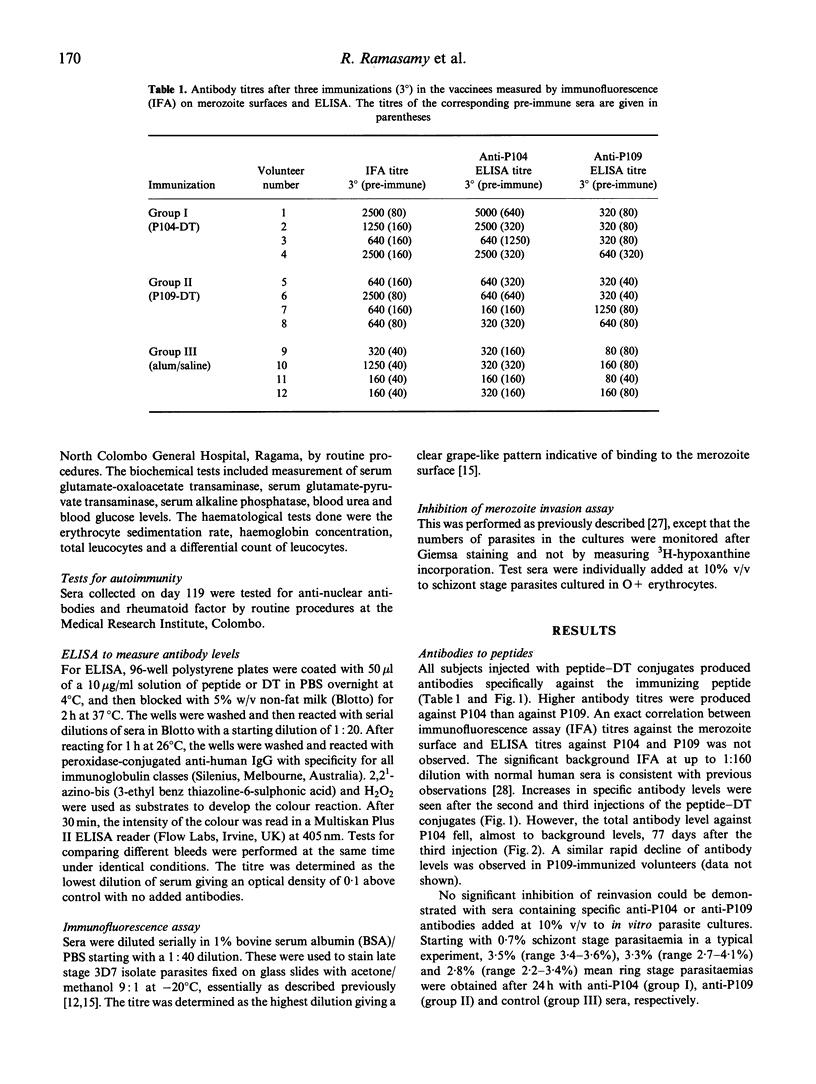
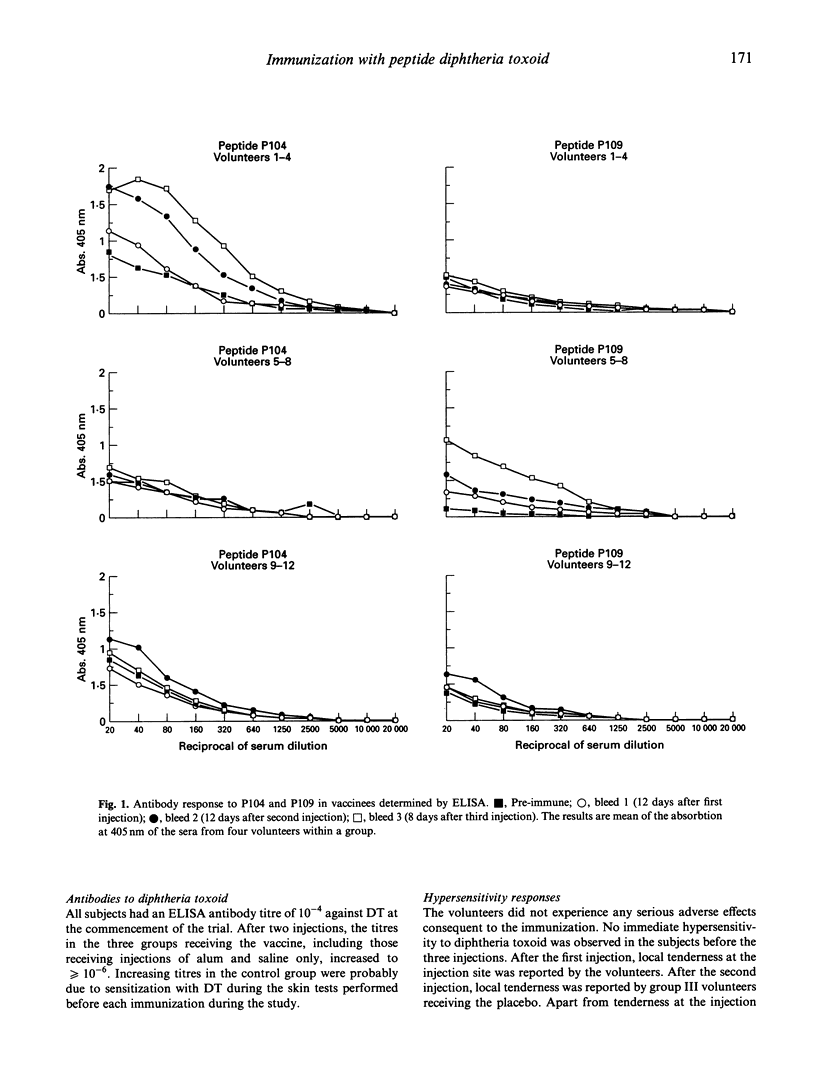
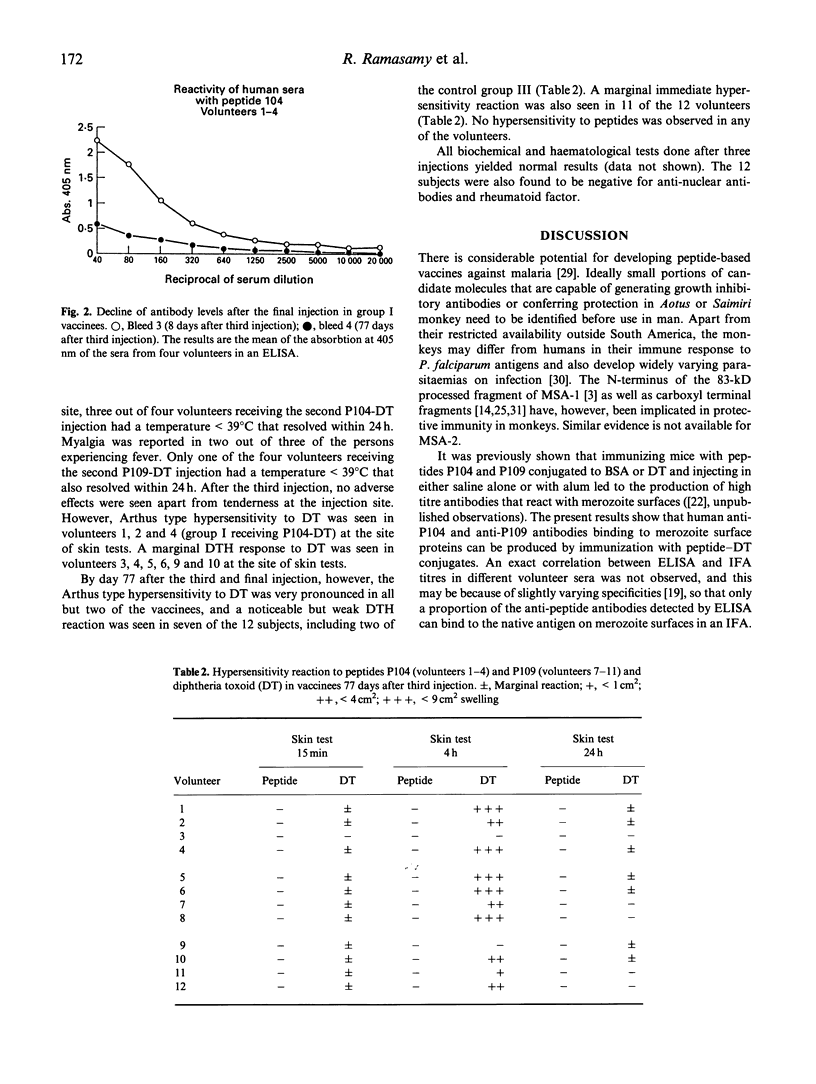
Twenty residue peptides from the 185-200-kD and 45-kD merozoite surface antigens of the malaria parasite Plasmodium falciparum were covalently linked to diphtheria toxoid as a carrier and used to immunize human volunteers with aluminium hydroxide as an adjuvant. Significant antibody levels were elicited by two boosting injections. The antibodies reacted with acetone-methanol fixed merozoite membranes in an immunofluorescence assay, but no inhibition of merozoite reinvasion could be detected in in vitro cultures containing the antibodies. Antibody levels against the immunizing peptides declined markedly within 77 days after the third injection. No hypersensitivity was observed against the peptides. However, the volunteers developed hypersensitivity against diphteria toxoid, and in particular a pronounced type III (Arthus) hypersensitivity after three injections with the toxoid. This effect might appear to limit the use of peptide-diphtheria toxoid conjugates for human immunization. Several biochemical, haematological and immunological tests done on the volunteers showed no other adverse effects from the immunizations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amador R., Moreno A., Valero V., Murillo L., Mora A. L., Rojas M., Rocha C., Salcedo M., Guzman F., Espejo F. The first field trials of the chemically synthesized malaria vaccine SPf66: safety, immunogenicity and protectivity. Vaccine. 1992;10(3):179–184. doi: 10.1016/0264-410x(92)90009-9. [DOI] [PubMed] [Google Scholar]
- Blackman M. J., Heidrich H. G., Donachie S., McBride J. S., Holder A. A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990 Jul 1;172(1):379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Gibson H. L., Lee-Ng C. T., Barr P. J., Hui G. S. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992 Jul 15;149(2):548–555. [PubMed] [Google Scholar]
- Etlinger H. M., Felix A. M., Gillessen D., Heimer E. P., Just M., Pink J. R., Sinigaglia F., Stürchler D., Takacs B., Trzeciak A. Assessment in humans of a synthetic peptide-based vaccine against the sporozoite stage of the human malaria parasite, Plasmodium falciparum. J Immunol. 1988 Jan 15;140(2):626–633. [PubMed] [Google Scholar]
- Etlinger H. M., Gillessen D., Lahm H. W., Matile H., Schönfeld H. J., Trzeciak A. Use of prior vaccinations for the development of new vaccines. Science. 1990 Jul 27;249(4967):423–425. doi: 10.1126/science.1696030. [DOI] [PubMed] [Google Scholar]
- Fandeur T., Dubois P., Gysin J., Dedet J. P., da Silva L. P. In vitro and in vivo studies on protective and inhibitory antibodies against Plasmodium falciparum in the Saimiri monkey. J Immunol. 1984 Jan;132(1):432–437. [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Nardin E. H., Losonsky G., Bathurst I. C., Barr P. J., Hollingdale M. R., Edelman R., Levine M. M. Safety and immunogenicity of a recombinant sporozoite malaria vaccine against Plasmodium vivax. Am J Trop Med Hyg. 1991 Dec;45(6):695–701. doi: 10.4269/ajtmh.1991.45.695. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. R., Bradley J., Judd S. J., Denholm E. H., Ing R. M., Mueller U. W., Powell J., Griffin P. D., Stevens V. C. Phase I clinical trial of a World Health Organisation birth control vaccine. Lancet. 1988 Jun 11;1(8598):1295–1298. doi: 10.1016/s0140-6736(88)92117-4. [DOI] [PubMed] [Google Scholar]
- Krchnák V., Mach O., Malý A. Computer prediction of B-cell determinants from protein amino acid sequences based on incidence of beta turns. Methods Enzymol. 1989;178:586–611. doi: 10.1016/0076-6879(89)78041-1. [DOI] [PubMed] [Google Scholar]
- Lee A. C., Powell J. E., Tregear G. W., Niall H. D., Stevens V. C. A method for preparing beta-hCG COOH peptide-carrier conjugates of predictable composition. Mol Immunol. 1980 Jun;17(6):749–756. doi: 10.1016/0161-5890(80)90145-5. [DOI] [PubMed] [Google Scholar]
- Lewis D. J., Castello-Branco L. R., Novotny P., Dougan G., Poulton T. A., Griffin G. E. Circulating cellular immune response to oral immunization of humans with cholera toxin B-subunit. Vaccine. 1993;11(2):119–121. doi: 10.1016/0264-410x(93)90005-i. [DOI] [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Heidrich H. G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987 Feb;23(1):71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- Millet P., Campbell G. H., Sulzer A. J., Grady K. K., Pohl J., Aikawa M., Collins W. E. Immunogenicity of the Plasmodium falciparum asexual blood-stage synthetic peptide vaccine SPf66. Am J Trop Med Hyg. 1993 Mar;48(3):424–431. doi: 10.4269/ajtmh.1993.48.424. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Ramasamy R. Binding of normal human immunoglobulins to Plasmodium falciparum. Indian J Med Res. 1988 Jun;87:584–593. [PubMed] [Google Scholar]
- Ramasamy R., Jones G., Lord R. Characterisation of an inhibitory monoclonal antibody-defined epitope on a malaria vaccine candidate antigen. Immunol Lett. 1990 Feb;23(4):305–309. doi: 10.1016/0165-2478(90)90077-4. [DOI] [PubMed] [Google Scholar]
- Ramasamy R., Nagendran K., Ramasamy M. S. Antibodies to epitopes on merozoite and sporozoite surface antigens as serologic markers of malaria transmission: studies at a site in the dry zone of Sri Lanka. Am J Trop Med Hyg. 1994 May;50(5):537–547. doi: 10.4269/ajtmh.1994.50.537. [DOI] [PubMed] [Google Scholar]
- Ramasamy R., Simpson R. J., Dexter A., Keeghan M., Reed C., Bushell G., Ingram L. T., Henderson T., Moloney M. B., Moritz R. L. Isolation and partial characterisation of a 26 kilodalton antigen from Plasmodium falciparum recognised by an inhibitory monoclonal antibody. Mol Biochem Parasitol. 1988 Jun;29(2-3):125–132. doi: 10.1016/0166-6851(88)90067-9. [DOI] [PubMed] [Google Scholar]
- Ramasamy R. Studies on glycoproteins in the human malaria parasite Plasmodium falciparum. Identification of a myristilated 45kDa merozoite membrane glycoprotein. Immunol Cell Biol. 1987 Oct;65(Pt 5):419–424. doi: 10.1038/icb.1987.48. [DOI] [PubMed] [Google Scholar]
- Ramasamy R. Synthetic peptides in malaria research. Pept Res. 1991 Jul-Aug;4(4):210–219. [PubMed] [Google Scholar]
- Ramasamy R., Wickremaratne C. Influence of N-terminal amino acids & conjugation position to carrier on specificities of antibodies elicited by malaria peptides. Indian J Med Res. 1994 Jan;99:21–26. [PubMed] [Google Scholar]
- Salcedo M., Barreto L., Rojas M., Moya R., Cote J., Patarroyo M. E. Studies on the humoral immune response to a synthetic vaccine against Plasmodium falciparum malaria. Clin Exp Immunol. 1991 Apr;84(1):122–128. doi: 10.1111/j.1365-2249.1991.tb08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Ruebush T. K., 2nd, Campbell G. H., Richman S. J., Wilkins P. P., Broderson J. R., Ardeshir F., Gross M., Silverman C., Skinner J. C. Immunogenicity and efficacy trials in Aotus nancymai monkeys with model compounds representing parts of a 75-kD merozoite surface antigen of Plasmodium falciparum. Am J Trop Med Hyg. 1992 Jun;46(6):691–707. doi: 10.4269/ajtmh.1992.46.691. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Smythe J. A., Coppel R. L., Brown G. V., Ramasamy R., Kemp D. J., Anders R. F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P., Lu Y. A. Vaccine engineering: enhancement of immunogenicity of synthetic peptide vaccines related to hepatitis in chemically defined models consisting of T- and B-cell epitopes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9084–9088. doi: 10.1073/pnas.86.23.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero M. V., Amador L. R., Galindo C., Figueroa J., Bello M. S., Murillo L. A., Mora A. L., Patarroyo G., Rocha C. L., Rojas M. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia. Lancet. 1993 Mar 20;341(8847):705–710. doi: 10.1016/0140-6736(93)90483-w. [DOI] [PubMed] [Google Scholar]
- Vreden S. G., Verhave J. P., Oettinger T., Sauerwein R. W., Meuwissen J. H. Phase I clinical trial of a recombinant malaria vaccine consisting of the circumsporozoite repeat region of Plasmodium falciparum coupled to hepatitis B surface antigen. Am J Trop Med Hyg. 1991 Nov;45(5):533–538. doi: 10.4269/ajtmh.1991.45.533. [DOI] [PubMed] [Google Scholar]


