Abstract
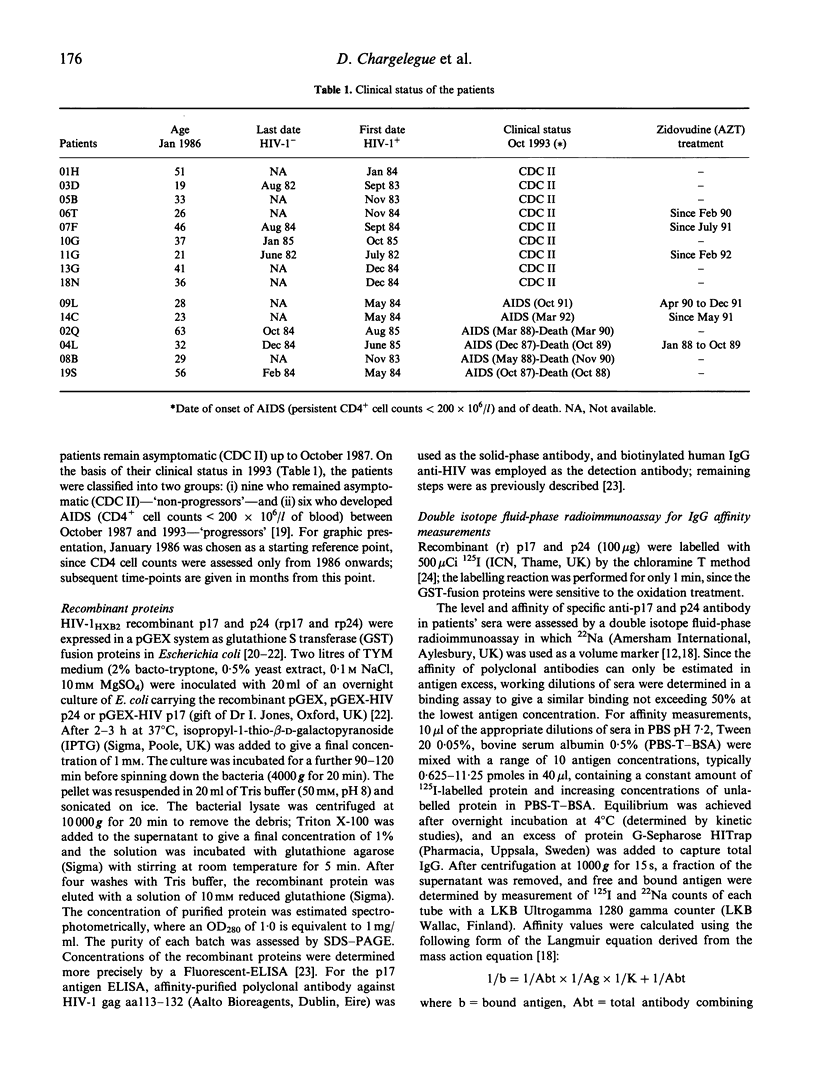
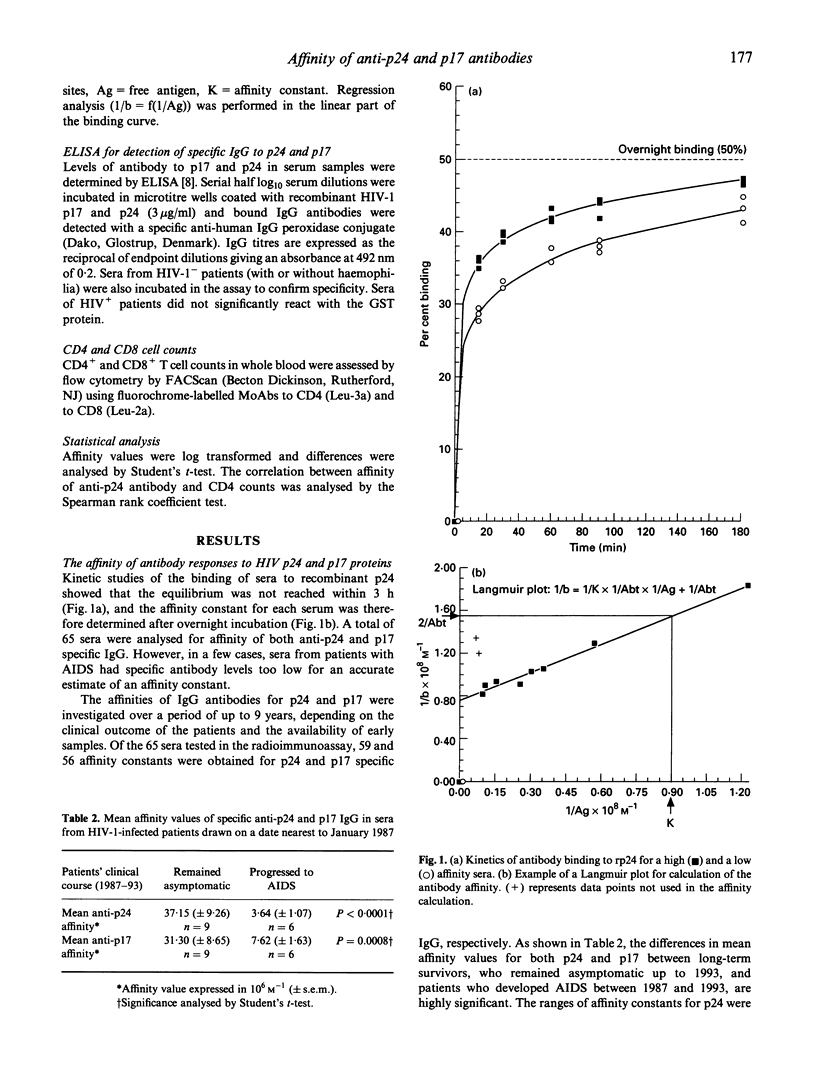
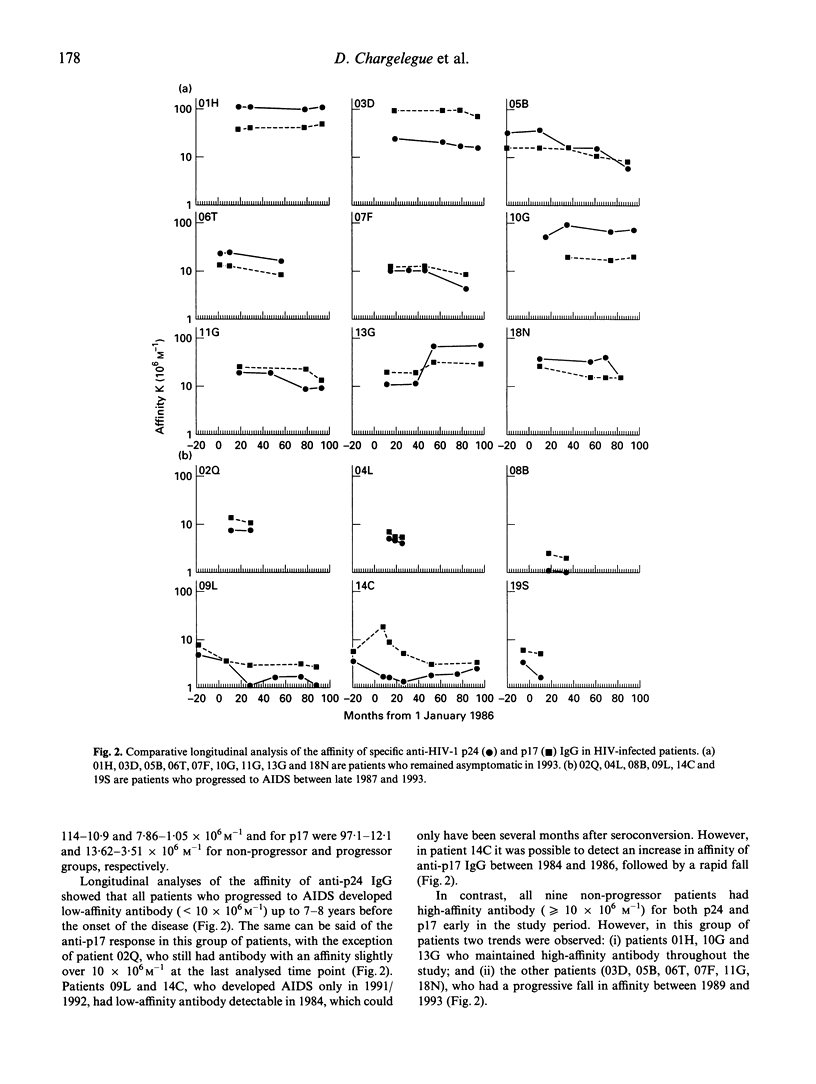
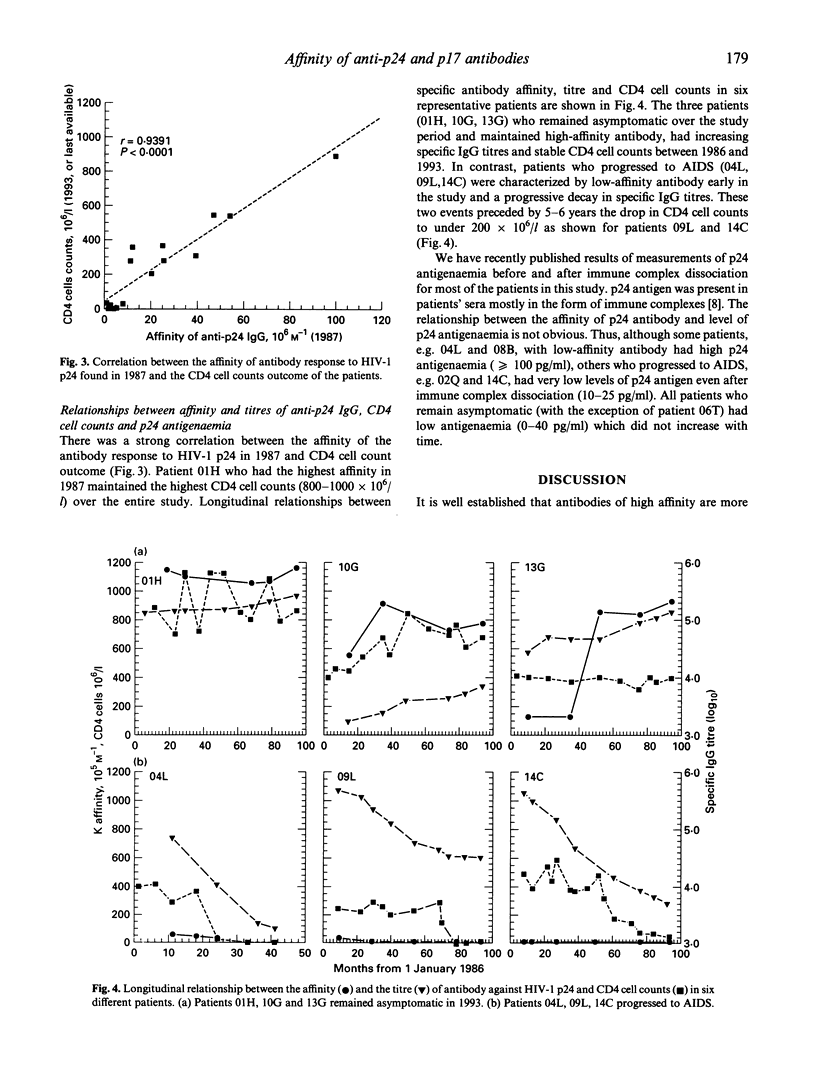
The affinity of anti-gag antibody was studied for up to 9 years (1984-1993) in sera from 15 HIV-1+ patients with haemophilia. On the basis of their 1993 clinical status patients were divided into two groups: (i) patients who remained asymptomatic (n = 9); and (ii) those who progressed to AIDS between late 1987 and 1993. The affinity constants of antibody for p24 and p17 were determined by a double isotope fluid-phase radioimmunoassay; and the relationships between antibody affinity and titre, patient clinical course, CD4 cell counts and p24 antigenaemia were analysed. The affinity of p24- and p17-specific antibody was up to 100 times greater in asymptomatic patients than in patients who progressed to AIDS. Patients who developed AIDS either lost or failed to develop high-affinity antibodies early in the infection. Asymptomatic patients maintained high-affinity antibodies for several years; however, in some of these patients the affinity of anti-p24 and p17 antibodies subsequently fell later in the study period. The presence of low-affinity antibody and progressive reduction in the titre of specific antibody were earlier predictors of disease onset than CD4 cell counts. The failure to either develop or maintain high affinity gag-specific antibody suggests an early impairment of T helper function in individuals who progressed to AIDS. The presence of antibody of high affinity could be essential in controlling virus replication and the onset of AIDS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Laurian Y., Einstein M. H., Braun B. P., Delaney S. R., Stephens J. E., Daluga C. K., Dahlen S. J., Knigge K. M. Monitoring of specific antibodies to human immunodeficiency virus structural proteins: clinical significance. Blood. 1991 Mar 1;77(5):1118–1123. [PubMed] [Google Scholar]
- Allain J. P., Laurian Y., Paul D. A., Verroust F., Leuther M., Gazengel C., Senn D., Larrieu M. J., Bosser C. Long-term evaluation of HIV antigen and antibodies to p24 and gp41 in patients with hemophilia. Potential clinical importance. N Engl J Med. 1987 Oct 29;317(18):1114–1121. doi: 10.1056/NEJM198710293171804. [DOI] [PubMed] [Google Scholar]
- Barin F., McLane M. F., Allan J. S., Lee T. H., Groopman J. E., Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985 May 31;228(4703):1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- Blank S. E., Leslie G. A., Clem L. W. Antibody affinity and valence in viral neutralization. J Immunol. 1972 Mar;108(3):665–673. [PubMed] [Google Scholar]
- Chargelegue D., Colvin B. T., O'Toole C. M. A 7-year analysis of anti-Gag (p17 and p24) antibodies in HIV-1-seropositive patients with haemophilia: immunoglobulin G titre and avidity are early predictors of clinical course. AIDS. 1993 Nov;7 (Suppl 2):S87–S90. doi: 10.1097/00002030-199311002-00017. [DOI] [PubMed] [Google Scholar]
- Chargelegue D., O'Toole C. M., Colvin B. T. A longitudinal study of the IgG antibody response to HIV-1 p17 gag protein in HIV-1+ patients with haemophilia: titre and avidity. Clin Exp Immunol. 1993 Sep;93(3):331–336. doi: 10.1111/j.1365-2249.1993.tb08181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargelegue D., O'Toole C. M. Development of a sensitive ELISA for HIV-1 p24 antigen using a fluorogenic substrate for monitoring HIV-1 replication in vitro. J Virol Methods. 1992 Aug-Sep;38(3):323–332. doi: 10.1016/0166-0934(92)90077-q. [DOI] [PubMed] [Google Scholar]
- Cheingsong-Popov R., Panagiotidi C., Bowcock S., Aronstam A., Wadsworth J., Weber J. Relation between humoral responses to HIV gag and env proteins at seroconversion and clinical outcome of HIV infection. BMJ. 1991 Jan 5;302(6767):23–26. doi: 10.1136/bmj.302.6767.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M. J., Lee T. H., Hatzakis A., Mandalaki T., McLane M. F., Essex M. Antibody responses in early human immunodeficiency virus type 1 infection in hemophiliacs. J Infect Dis. 1988 Apr;157(4):805–811. doi: 10.1093/infdis/157.4.805. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Zanotto C., Mammano F., Ometto L., Del Mistro A., Chieco-Bianchi L. Pattern of antibody response against the V3 loop in children with vertically acquired immunodeficiency virus type 1 (HIV-1) infection. AIDS Res Hum Retroviruses. 1993 Mar;9(3):221–228. doi: 10.1089/aid.1993.9.221. [DOI] [PubMed] [Google Scholar]
- Devash Y., Calvelli T. A., Wood D. G., Reagan K. J., Rubinstein A. Vertical transmission of human immunodeficiency virus is correlated with the absence of high-affinity/avidity maternal antibodies to the gp120 principal neutralizing domain. Proc Natl Acad Sci U S A. 1990 May;87(9):3445–3449. doi: 10.1073/pnas.87.9.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Frank M. M., Johnson J. S. The relationship between antibody affinity and the efficiency of complement fixation. J Immunol. 1970 Jul;105(1):215–220. [PubMed] [Google Scholar]
- Fenouillet E., Blanes N., Coutellier A., Demarquest J., Rozenbaum W., Gluckman J. C. Monitoring of antibodies against human immunodeficiency virus type 1 p25 core protein as prognostic marker. J Infect Dis. 1992 Sep;166(3):611–616. doi: 10.1093/infdis/166.3.611. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Holland G. P., Steward M. W. Antibody affinity maturation: the role of CD8+ cells. Clin Exp Immunol. 1989 Dec;78(3):488–493. [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills H. R., Berry N., Burns N. R., Jones I. M. Simple and efficient production of the core antigens of HIV-1, HIV-2 and simian immunodeficiency virus using pGEX expression vectors in Escherichia coli. AIDS. 1992 Apr;6(4):437–439. [PubMed] [Google Scholar]
- Nara P. L., Garrity R. R., Goudsmit J. Neutralization of HIV-1: a paradox of humoral proportions. FASEB J. 1991 Jul;5(10):2437–2455. doi: 10.1096/fasebj.5.10.1712328. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Partidos C., Stanley C., Steward M. The influence of orientation and number of copies of T and B cell epitopes on the specificity and affinity of antibodies induced by chimeric peptides. Eur J Immunol. 1992 Oct;22(10):2675–2680. doi: 10.1002/eji.1830221030. [DOI] [PubMed] [Google Scholar]
- Reid G., Rigby M. A., McDonald M., Hosie M. J., Neil J. C., Jarrett O. Immunodiagnosis of feline immunodeficiency virus infection using recombinant viral p17 and p24. AIDS. 1991 Dec;5(12):1477–1483. doi: 10.1097/00002030-199112000-00010. [DOI] [PubMed] [Google Scholar]
- Robertson C. A., Mok J. Y., Froebel K. S., Simmonds P., Burns S. M., Marsden H. S., Graham S. Maternal antibodies to gp120 V3 sequence do not correlate with protection against vertical transmission of human immunodeficiency virus. J Infect Dis. 1992 Oct;166(4):704–709. doi: 10.1093/infdis/166.4.704. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Clerici M. Early T-helper cell defects in HIV infection. AIDS. 1991 Mar;5(3):245–253. doi: 10.1097/00002030-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Stanley C. M., Dimarchi R., Mulcahy G., Doel T. R. High-affinity antibody induced by immunization with a synthetic peptide is associated with protection of cattle against foot-and-mouth disease. Immunology. 1991 Jan;72(1):99–103. [PMC free article] [PubMed] [Google Scholar]
- Underwood P. A. Problems and pitfalls with measurement of antibody affinity using solid phase binding in the ELISA. J Immunol Methods. 1993 Aug 26;164(1):119–130. doi: 10.1016/0022-1759(93)90282-c. [DOI] [PubMed] [Google Scholar]
- VanCott T. C., Bethke F. R., Polonis V. R., Gorny M. K., Zolla-Pazner S., Redfield R. R., Birx D. L. Dissociation rate of antibody-gp120 binding interactions is predictive of V3-mediated neutralization of HIV-1. J Immunol. 1994 Jul 1;153(1):449–459. [PubMed] [Google Scholar]
- Wen Y. M., Duan S. C., Howard C. R., Frew A. F., Steward M. W. The affinity of anti-HBc antibodies in acute and chronic hepatitis B infection. Clin Exp Immunol. 1990 Jan;79(1):83–86. doi: 10.1111/j.1365-2249.1990.tb05131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., Nara P. L., Goudsmit J. Genotypic and phenotypic variation of HIV-1: impact on AIDS pathogenesis and vaccination. Chem Immunol. 1993;56:1–33. [PubMed] [Google Scholar]
- Zinkernagel R. M., Hengartner H. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol Today. 1994 Jun;15(6):262–268. doi: 10.1016/0167-5699(94)90005-1. [DOI] [PubMed] [Google Scholar]


