Abstract
Introduction
Under conditions of shock, bacteria and endotoxins in the intestines can traverse the mucosal barrier by translocation and enter the blood and lymphatic system. Immunoglobulins and lactoferrin have been reported to neutralize endotoxins and bacteria. We studied the essential therapeutic factors of colostrum products in an animal experiment.
Method
We simulated endotoxaemia by per-oral administration of a suspension of Escherichia coli and antibiotics into the duodenum of anaesthetized rats after giving intraperitoneal carrageenan. At the same time, pure bovine colostrum or lactoferrin-enriched bovine colostrum was given. Therapeutic effects were studied by examining plasma endotoxin activity and bacterial contamination of mesenterial lymph nodes and peritoneal lavages. Albumin was used in a control group.
Results
The most effective bovine colostrum was able to reduce the maximum plasma endotoxin value by 67% as compared with the albumin group. The combination of this colostrum with lactoferrin brought about a reduction by 80%. The reduction in bacterial contamination of lymph nodes and peritoneal lavages was also evident.
Conclusion
Both gammaglobulin and lactoferrin may help to eliminate endotoxins when bovine colostrum is administered into the gut in conditions of septic shock.
Keywords: colostrum, endotoxaemia, experimental septic shock
Introduction
Septic shock is a frequent cause of death in intensive care medicine. Possible translocation of bacteria and endotoxins renders the gastrointestinal tract a crucial factor in this condition [1]. Reduced perfusion of the splanchnic region because of centralization results in hypoxia and oedema of the intestinal mucosa. The protective function of the gastrointestinal tract breaks down and bacteria from the gut enter the blood and lymph system [2].
Per-oral administration of bovine milk immunoglobulin has been proved to be effective in the treatment of intestinal Escherichia coli infection [3]. The biological activity of bacteria and endotoxins was reduced [4,5]. The present study was conducted to determine whether those findings could be confirmed in vivo by using three different bovine colostrum products in animal experiments.
Materials and method
The animal experiments were approved by the Animal Care Committee (Ethikkommission) of the University Hospital of Kiel. The colostrum products were provided by the Institut für Chemie und Physik, Bundesanstalt für Milchforschung, Kiel, and were not contaminated with lipopolysaccharide.
A total of 35 male Wistar rats (250–350 g) were anaesthetized with ketamine. In order to achieve a degree of immunosuppression (i.e. an inflammatory state [6]) in the gut and a higher initial level of plasma endotoxin activity, 80 mg of type IV carrageenan/kg (Sigma-Aldrich Corp., St Louis, MO, USA) was injected into the peritoneal cavity. The animals were randomly assigned to five groups, each comprising seven animals (Table 1): groups 1, 2 and 3 received 400 mg iron-saturated bovine colostrum/kg of different compositions (i.e. types 1–3 bovine colostra); group 4 received a combination of 400 mg type 2 bovine colostrum/kg plus 80 mg bovine iron-saturated lactoferrin/kg (Sigma); and group 5 received 400 mg human albumin/kg (control substance).
Table 1.
Composition of bovine colostrum types 1, 2 and 3, and lactoferrin
| Substance | α-Lacto-albumin (g/dl) | β-Lactoglobulin (g/dl) | Bovine serum albumin (g/dl) | Immunoglobulins (g/dl) | LF (g/dl) | Casein (g/dl) | Iron (g/dl) |
| Type 1 BC | 4400 | 44,400 | 3600 | 79,600 | 1600 | ND | 200 |
| Type 2 BC | 4000 | 22,400 | 1600 | 151,600 | ND | 80,000 | 90 |
| Type 3 BC | 2800 | 14,000 | 2400 | 145,600 | ND | 80,000 | 200 |
| LF | ND | ND | ND | ND | 80,000 | ND | 125 |
BC, bovine colostrum; LF, lactoferrin; ND, not detected.
As albumin, like other proteins, has an unspecific endotoxin-binding capacity, we wanted to have comparable protein quantities as referred to a protein commonly used in intensive care medicine. At the beginning of the 5-hour period of observation, the first blood samples were taken from the exposed external jugular vein. Neomycin and bacitracin are bactericidal and stimulate release of lipopolysaccharide from E. coli, and their administration results in increasing plasma endotoxin levels. Therefore, a combination of 5 × 1010 colony-forming units of E. coli/kg (strain O:NT H16 clinical isolate; University of Kiel, Germany); 10 mg neomycin and bacitracin/kg; and the group-specific colostrum or albumin was administered through a per-oral tube (diameter 2 mm). The tube was fixed in the duodenum by laparotomy (L.N.), carefully avoiding damage to the efferent biliary tract.
Preliminary investigations without carrageenan had shown that the maximum endotoxin level in plasma (45 ± 4 EU/dl) was reached 5 hours after administration of the bacteria/neomycin–bacitracin suspension. Therefore, the plasma endotoxin activity was measured hourly for 5 hours. At the same time points, 2 ml blood was taken from the jugular vein for culture. The last assessment was followed by laparotomy (L.N.) and peritoneal lavages with 10 ml endotoxin-free 0.9% saline solution.
Furthermore, mesenteric lymph nodes from four different areas of the mesenterium (duodenum and colon) were resected and homogenized. Bacterial contamination of the lymph nodes and the hourly blood cultures were examined using smears on sheep blood agar plates incubated for 48 hours at 37°C. Each lymph node area was examined on one agar plate and the peritoneal lavage on three agar plates. Quantity and specification of the bacterial contamination were not assessed and no anaerobic cultures were performed.
In order to measure the biological endotoxin activity in serum, we used the modified Limulus amoebocyte/lysate test with a chromogenic substrate [7]. First, 100 l of a 1:40 diluted (0.9% NaCl) plasma sample was heated for 5 min at 80°C. Then 50 μl of Limulus lysate (Pyroquant 50, ChB: 42-109-551; Pyroquant Diagnostik GmbH, Mörfelden-Walldorf, Germany) was added and incubated for 45 min at 37°C. inally 100 l of chromogenic substrate (S-2423; Chromogenic Company, Mölndal, Sweden) was added and incubated for 4 min at 37°C. The reaction was stopped with 50 l acetic acid (100%) and the extinction was measured by a photometer at a wavelength of 405 nm. The lower limit of detection is 3 EU/dl. The baseline plasma endotoxin activity without application of the bacteria/neomycin–bacitracin suspension was below the lower limit of detection.
Statistical analysis of plasma endotoxin activity was based on the arithmetic means and standard deviations. Statistical significance was evaluated using the nonparametric (distribution-free method) U test (Wilcoxon and Mann–Whitney). P < 0.05 was considered statistically significant.
Results
Starting from a control value just above the limit of detection, there was an approximately linear rise in plasma endotoxin up to the 4-hour value of 132 ± 29 EU/dl in group 5. The 5-hour value of 135 ± 24 EU/dl was only slightly higher (Fig. 1). The bovine colostra administered in groups 1 and 3 significantly lowered biological activity from the 1-hour value onward (Table 2). The most effective suppression of biological activity was observed in group 2, in which the maximum plasma endotoxin value was 60 ± 20 EU/dl after 3 hours. At the end of the observation period the value was just 44 ± 10 EU/dl. This amounts to a reduction of 67.3% (Fig. 1). In group 4 endotoxin activity was reduced to 27 ± 7 EU/dl (i.e. a maximum reduction of 80%; Fig. 1).
Figure 1.
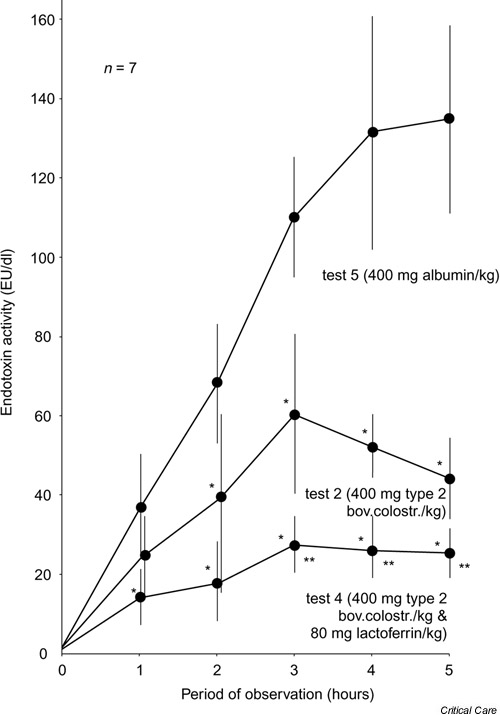
Mean values and standard deviations of plasma endotoxin activity in experimental group 2 (test 2) and 4 (test 4), and in control group 5 (test 5). *P < 0.05, versus control group; **P < 0.05, versus group 2.
Table 2.
Plasma endotoxin activity
| Time (h) | Albumin (EU/dl) | BC type 1 | BC type 3 |
| 0 | 3.0 ± 1.0 | 2.0 ± 1.0 | 3.0 ± 1.1 |
| 1 | 36.0 ± 15.1 | 18 ± 2.9* | 10.0 ± 4.0* |
| 2 | 69.0 ± 17.2 | 30.0 ± 5.0* | 18.0 ± 2.5* |
| 3 | 110.0 ± 15.7 | 63.0 ± 21.7* | 40.1 ± 5.0* |
| 4 | 132.0 ± 29.3 | 87.3 ± 26* | 62.0 ± 10.7* |
| 5 | 135.0 ± 24.0 | 75.2 ± 22.8* | 52.0 ± 3.0* |
Shown are mean values and standard deviations of plasma endotoxin activity for bovine colostrum (BC) types 1 and 3 (400 mg/kg each) as compared with the albumin control group (400 mg/kg). Each group contained seven rats. *P < 0.05, versus control group.
Bacterial contamination of the peritoneal lavages and lymph nodes was found to be lowest in experimental group 4 (Table 3). There was no bacterial contamination in any blood culture after incubation for 48 hours at 37°C.
Table 3.
Bacterial contamination after 48 hours of incubation of peritoneal lavage and lymph node specimens
| Substance | Positive lavage (%) | Positive lymph nodes (%) |
| Albumin | 62.5 | 62.5 |
| Type 1 BC | 27.3* | 48.0* |
| Type 2 BC | 57.0 | 55.3 |
| Type 3 BC | 25.0* | 20.5* |
| Type 2 BC + 80 mg LF/kg | 18.0* | 17.0* |
Shown are findings with bovine colostrum (BC) types 1, 2 and 3 (400 mg/kg each), and BC type 2 with 80 mg/kg lactoferrin (LF) as compared with the albumin control group (400 mg/kg). *P < 0.05, versus control group.
Discussion
The gastrointestinal tract is of great importance for the development and prognosis of septicaemia [1,2]. The course of intensive care patients could be influenced favourably by selective decontamination of the intestine with local antibiotic therapy [8]. However, such bactericidal preparations can liberate lipid A fragments from the bacterial cell wall and thus increase the translocation of endotoxin [9]. It would therefore appear rational to combine antibiotic with a substance that inactivates both bacteria and endotoxins [10,11]. An oral dietetic would be of particular importance in this regard because plain parenteral nutrition lowers the concentration of secretory IgA in bile. This weakens immunological resistance and thus diminishes the barrier function of the intestinal mucosa [12]. We attempted to demonstrate that bovine colostrum is better able to inactivate lipopolysaccharide than albumin.
Administration of bovine colostrum has already proven efficacious in treating bacterial and viral enteritis in babies and infants [13]. Enteral administration of bovine colostrum with a high immunoglobulin content has been found to reduce peri-operative translocation of endotoxin from the gastrointestinal tract [14]. However, it is still unclear which constituents of bovine colostrum are the crucial biological factors in this therapeutic effect. Neutralization of endotoxins and bacteria has been reported for immunoglobulins and lactoferrin [5,14].
In our animal experiments, group 2 exhibited the greatest suppression of plasma endotoxin level. This may be due to the high immunoglobulin content. The reduction in endotoxin activity was also significant in group 1. Because that colostrum contains only half as much immunoglobulin as the colostra in groups 2 and 3, a distinct effect of lactoferrin in group 1 is possible. The iron-saturation of the preparations rules out the possibility that the bacteriostatic effect of lactoferrin derives from removal of iron from the bacterial cell wall. Therefore, lactoferrin also confers a specific defence mechanism. Intensified elimination of the endotoxin by 'natural killer cells' is conceivable because iron-saturated lactoferrin can activate these cells. The positive therapeutic effect of combining type 2 colostrum with 80 mg lactoferrin/kg supports this.
Corresponding effects were evident in the lymph nodes and peritoneal lavages. The role of systemic and local proinflammatory cytokine levels and the significance of immunoglobulins and lactoferrin [4,15,16] in such animal models should be studied in further research. Because measurement of endotoxin in biological fluids is difficult, new techniques such as the endotoxin activity assay should be considered [17].
Competing interests
None declared.
Key messages
• Enteral application of colostrum products is useful in septic shock
• Colostra with a high amount of lactoferrin can reduce both endotoxin activity in plasma and bacterial contamination of the peritoneal cavity
• Colostra and lactoferrin may help to improve outcomes in treatment of septic patients
Acknowledgments
Acknowledgement
The present report is a partial extract of a doctoral thesis by L.N. (University of Kiel, Germany).
References
- Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990;18:529–536. doi: 10.1097/00003246-199005000-00014. [DOI] [PubMed] [Google Scholar]
- Saadia R, Schein M, MacFarlane C, Boffard KD. Gut barrier function and the surgeon. Br J Surg. 1990;77:487–492. doi: 10.1002/bjs.1800770505. [DOI] [PubMed] [Google Scholar]
- Brunser O, Espinoza J, Figueroa G, Araya M, Spencer E, Hilpert H, Link-Amster H, Brussow H. Field trial of an infant formula containing anti-rotavirus and anti-Escherischia coli milk antibodies from hyperimmunized cows. J Pediatr Gastroenterol Nutr. 1992;15:63–72. doi: 10.1097/00005176-199207000-00010. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Tazume S, Shimizu K, Matsuzawa H, Dosako S, Isoda H, Tsukiji M, Fujimura R, Muranaka Y, Isihida H. Inhibitory effects of bovine lactoferrin on the adherence of enterotoxigenic Escherichia coli to host cells. Biosci Biotechnol Biochem. 2000;64:348–354. doi: 10.1271/bbb.64.348. [DOI] [PubMed] [Google Scholar]
- Lissner R, Schmidt H, Karch H. A standard immunoglobulin preparation produced from bovine colostra shows antibody reactivity and neutralization activity against Shiga-like toxins and EHEC-hemolysin of Escherischia coli 0157:H7. Infection. 1996;24:378–383. doi: 10.1007/BF01716084. [DOI] [PubMed] [Google Scholar]
- Pricolo VE, Madhere SM, Finkelstein SD, Reichner JS. Effects of lambda-carrageenan induced experimental enterocolitis on splenocyte function and nitric oxide production. J Surg Res. 1996;66:6–11. doi: 10.1006/jsre.1996.0364. [DOI] [PubMed] [Google Scholar]
- Harris RI, Stone PC, Stuart J. An improved chromogenic substrate endotoxin assay for clinical use. J Clin Pathol. 1983;36:1145–1149. doi: 10.1136/jcp.36.10.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Saene HK, Stoutenbeek CP, Zandstra DF. Selective decontamination of the digestive tract (SDD) in multiple trauma patients. J Trauma. 1998;44:570–572. doi: 10.1097/00005373-199803000-00034. [DOI] [PubMed] [Google Scholar]
- Prins JM, van Deventer SJ, Kuijper EJ, Speelman P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob Agents Chemother. 1994;38:1211–1218. doi: 10.1128/aac.38.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean-Nystrom EA, Pohlenz JF, Moon HW, O'Brian AD. Escherichia coli O157:H7 causes more-severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect Immun. 2000;68:2356–2358. doi: 10.1128/IAI.68.4.2356-2358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warny M, Fatimi A, Bostwick EF, Laine DC, Lebel F, LaMont JT, Pothoulakis C, Kelly CP. Bovine immunoglobulin concentrate-Clostridium difficile retains C difficile toxin neutralising activity after passage through the human stomach and small intestine. Gut. 1999;44:212–217. doi: 10.1136/gut.44.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DJ, Alverdy JC, Aoys E, Moss GS. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989;124:1396–1399. doi: 10.1001/archsurg.1989.01410120042009. [DOI] [PubMed] [Google Scholar]
- Feist N, Berger D, Speer CP. Anti-endotoxin antibodies in human milk: correlation with infection of the newborn. Acta Paediatr. 2000;89:1087–1092. doi: 10.1080/713794575. [DOI] [PubMed] [Google Scholar]
- Raqib R, Mia SM, Qadri F, Alam TI, Alam NH, Chowdhury AK, Mathan MM, Andersson J. Innate immune responses in children and adults with Shigellosis. Infect Immun. 2000;68:3620–3629. doi: 10.1128/IAI.68.6.3620-3629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, MacLaren DM, de Graaf J, Nuijens JH. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebermann L, Döhler JR, Perlick L. Treatment of enterogenic endotoxinemia with lactoferrin in rats. Langenbeck's Arch Surg. 2000;386:146–149. doi: 10.1007/s004230000191. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care. 2002;6:342–348. doi: 10.1186/cc1522. [DOI] [PMC free article] [PubMed] [Google Scholar]


