Abstract
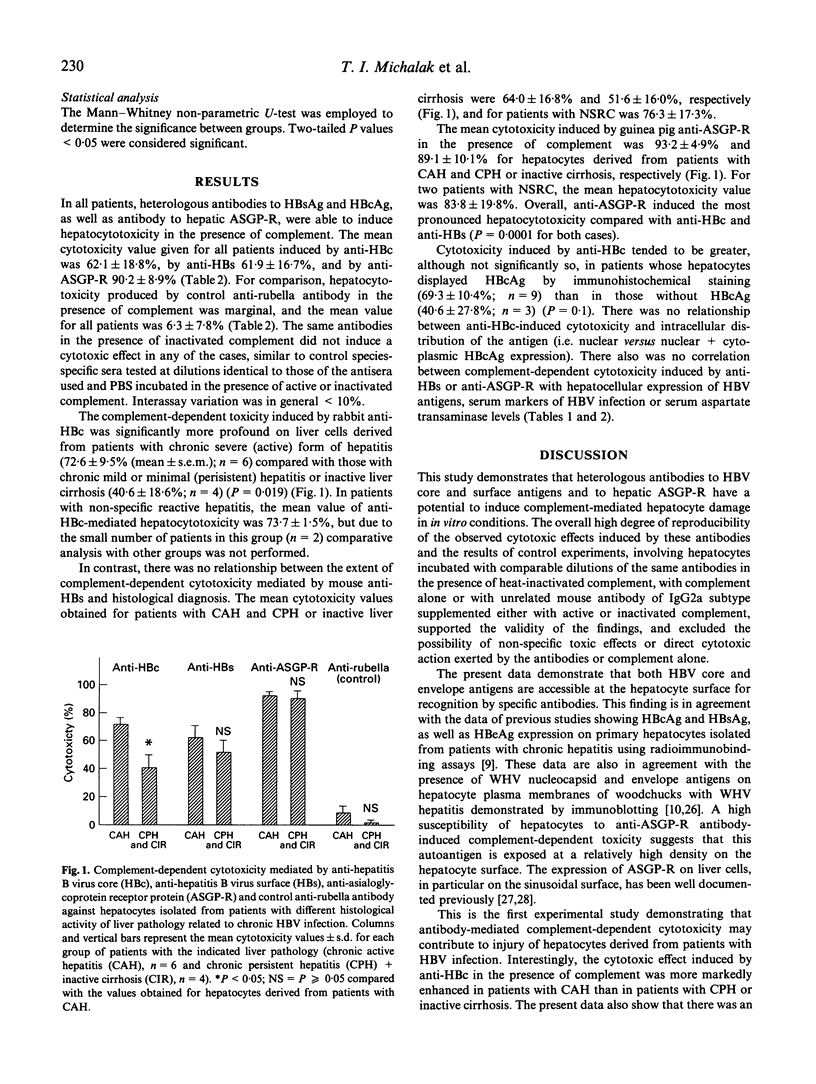
The susceptibility of hepatocytes from patients with chronic hepatitis B to complement-dependent cytotoxicity mediated by heterologous antibodies to hepatitis B virus core (anti-HBc) and surface (anti-HBs) antigens and to hepatic asialoglycoprotein receptor was examined using a microcytotoxicity assay. The anti-HBc-induced cytotoxicity was found to be markedly enhanced against hepatocytes isolated from patients with chronic active hepatitis (72.6 +/- 9.5% (mean +/- s.e.m.); n = 6) over that against hepatocytes from individuals with chronic persistent hepatitis or inactive liver cirrhosis (40.6 +/- 18.6%; n = 4) (P = 0.019). Overall, values of the anti-HBc-directed cytotoxicity were higher in patients positive for HBcAg in hepatocytes and seropositive for hepatitis B virus e antigen (HBeAg). Hepatocytotoxicity was also exerted by anti-HBs and anti-asialoglycoprotein receptor antibodies in the presence of complement, but it was not seemingly related to disease activity. These results indicate that hepatitis B virus core and surface antigens and asialoglycoprotein receptor at the hepatocyte surface can be recognized by antibodies, and raise the possibility that complement-dependent cytolysis may contribute to the injury of hepatitis B virus-infected hepatocytes. The data also suggest that liver cells of patients with severe chronic hepatitis might be more susceptible to anti-HBc antibody-directed complement-mediated cytotoxicity than those with inactive liver histology.
Full text
PDF

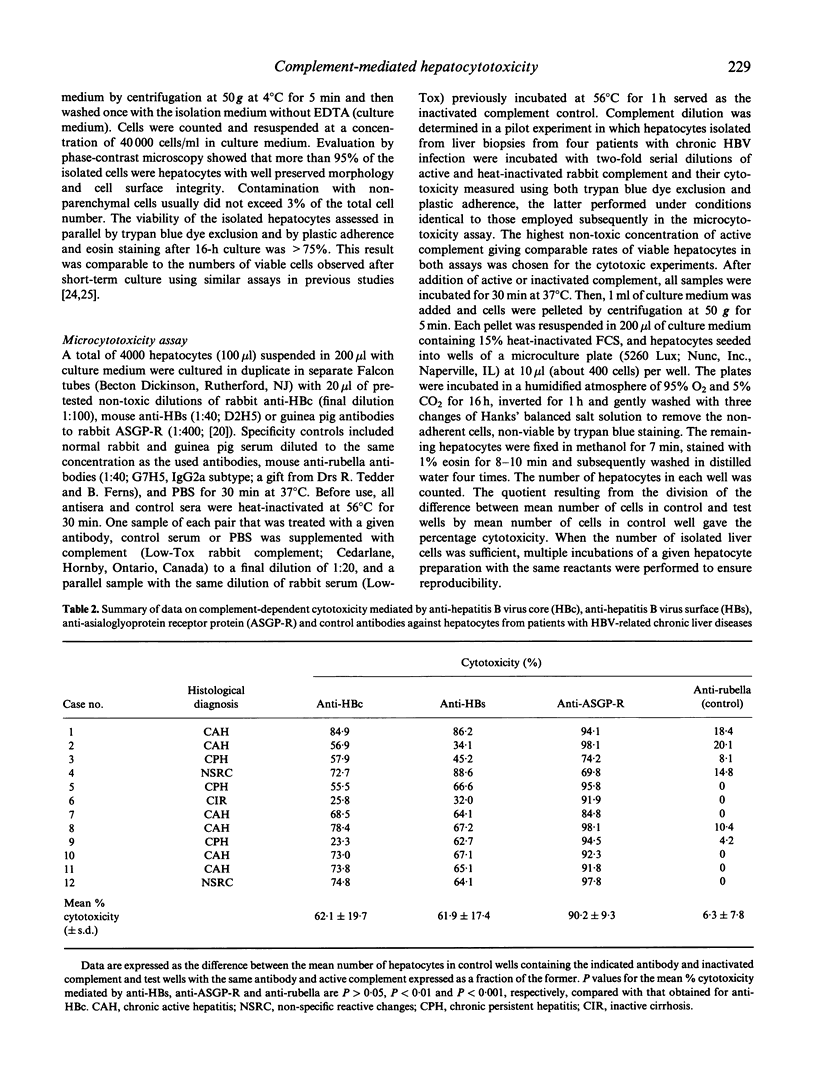


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Bertoletti A., Ferrari C., Fiaccadori F., Penna A., Margolskee R., Schlicht H. J., Fowler P., Guilhot S., Chisari F. V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H. E., Liang T. J., Galun E., Wands J. R. Persistence of hepatitis B viral DNA after serological recovery from hepatitis B virus infection. Hepatology. 1991 Jul;14(1):56–63. doi: 10.1002/hep.1840140110. [DOI] [PubMed] [Google Scholar]
- Daniels C. K., Smith K. M., Schmucker D. L. Asialoorosomucoid hepatobiliary transport is unaltered by the loss of liver asialoglycoprotein receptors in aged rats. Proc Soc Exp Biol Med. 1987 Nov;186(2):246–250. doi: 10.3181/00379727-186-2-rc1. [DOI] [PubMed] [Google Scholar]
- Dzwonkowski P., Michalak T. I. Autoantibody pattern in a woodchuck model of hepatitis B. Clin Invest Med. 1990 Dec;13(6):322–328. [PubMed] [Google Scholar]
- Fagan E. A., Guarner P., Perera S. D., Trowbridge R., Rolando N., Davison F., Williams R. Quantitation of hepatitis B virus DNA (HBV DNA) in serum using the spot hybridization technique and scintillation counting. J Virol Methods. 1985 Dec;12(3-4):251–262. doi: 10.1016/0166-0934(85)90136-3. [DOI] [PubMed] [Google Scholar]
- Harford J., Ashwell G. G. Immunological evidence for the transmembrane nature of the rat liver receptor for asialoglycoproteins. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1557–1561. doi: 10.1073/pnas.78.3.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle J. H. Serologic markers of hepatitis B virus infection. Annu Rev Med. 1981;32:1–11. doi: 10.1146/annurev.me.32.020181.000245. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Kurebayashi Y. A rat model of acute liver necrosis induced by a monoclonal antibody to liver-specific antigen and complement. Hepatology. 1991 Jun;13(6):1152–1157. [PubMed] [Google Scholar]
- Krugman S., Overby L. R., Mushahwar I. K., Ling C. M., Frösner G. G., Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med. 1979 Jan 18;300(3):101–106. doi: 10.1056/NEJM197901183000301. [DOI] [PubMed] [Google Scholar]
- Lau J. Y., Bain V. G., Davies S. E., Alexander G. J., Williams R. Export of intracellular HBsAg in chronic hepatitis B virus infection is related to viral replication. Hepatology. 1991 Sep;14(3):416–421. [PubMed] [Google Scholar]
- Lau J. Y., Bain V. G., Naoumov N. V., Smith H. M., Alexander G. J., Williams R. Effect of interferon-gamma on hepatitis B viral antigen expression in primary hepatocyte culture. Hepatology. 1991 Dec;14(6):975–979. [PubMed] [Google Scholar]
- Lau J. Y., Bird G. L., Naoumov N. V., Williams R. Hepatic HLA antigen display in chronic hepatitis B virus infection. Relation to hepatic expression of HBV genome/gene products and liver histology. Dig Dis Sci. 1993 May;38(5):888–895. doi: 10.1007/BF01295916. [DOI] [PubMed] [Google Scholar]
- Löhr H., Treichel U., Poralla T., Manns M., Meyer zum Büschenfelde K. H., Fleischer B. The human hepatic asialoglycoprotein receptor is a target antigen for liver-infiltrating T cells in autoimmune chronic active hepatitis and primary biliary cirrhosis. Hepatology. 1990 Dec;12(6):1314–1320. doi: 10.1002/hep.1840120611. [DOI] [PubMed] [Google Scholar]
- McFarlane B. M., McSorley C. G., Vergani D., McFarlane I. G., Williams R. Serum autoantibodies reacting with the hepatic asialoglycoprotein receptor protein (hepatic lectin) in acute and chronic liver disorders. J Hepatol. 1986;3(2):196–205. doi: 10.1016/s0168-8278(86)80026-5. [DOI] [PubMed] [Google Scholar]
- McFarlane B. M., Sipos J., Gove C. D., McFarlane I. G., Williams R. Antibodies against the hepatic asialoglycoprotein receptor perfused in situ preferentially attach to periportal liver cells in the rat. Hepatology. 1990 Mar;11(3):408–415. doi: 10.1002/hep.1840110312. [DOI] [PubMed] [Google Scholar]
- McFarlane I. G. Autoimmunity and hepatotropic viruses. Semin Liver Dis. 1991 Aug;11(3):223–233. doi: 10.1055/s-2008-1040440. [DOI] [PubMed] [Google Scholar]
- Michalak T. I., Lin B., Churchill N. D., Dzwonkowski P., Desousa J. R. Hepadna virus nucleocapsid and surface antigens and the antigen-specific antibodies associated with hepatocyte plasma membranes in experimental woodchuck acute hepatitis. Lab Invest. 1990 Jun;62(6):680–689. [PubMed] [Google Scholar]
- Michalak T. I., Lin B. Molecular species of hepadnavirus core and envelope polypeptides in hepatocyte plasma membrane of woodchucks with acute and chronic viral hepatitis. Hepatology. 1994 Aug;20(2):275–286. [PubMed] [Google Scholar]
- Michalak T. I., Pasquinelli C., Guilhot S., Chisari F. V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994 Jan;93(1):230–239. doi: 10.1172/JCI116950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M., Kloppel T. M., Nakane P. K., Brown W. R., Vierling J. M. Cellular distribution of the asialoglycoprotein receptor in rat liver. Implications for hepatic accumulation of desialylated lymphocytes. Gastroenterology. 1984 Jan;86(1):142–149. [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Naumov N. V., Mondelli M., Alexander G. J., Tedder R. S., Eddleston A. L., Williams R. Relationship between expression of hepatitis B virus antigens in isolated hepatocytes and autologous lymphocyte cytotoxicity in patients with chronic hepatitis B virus infection. Hepatology. 1984 Jan-Feb;4(1):63–68. doi: 10.1002/hep.1840040111. [DOI] [PubMed] [Google Scholar]
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H. J., Vitiello A., Chesnut R., Person J. L., Redeker A. G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993 May 15;150(10):4659–4671. [PubMed] [Google Scholar]
- Nowoslawski A., Krawczyński K., Brzosko W. J., Madaliński K. Tissue localization of Australia antigen immune complexes in acute and chronic hepatitis and liver cirrhosis. Am J Pathol. 1972 Jul;68(1):31–56. [PMC free article] [PubMed] [Google Scholar]
- Peters M., Vierling J., Gershwin M. E., Milich D., Chisari F. V., Hoofnagle J. H. Immunology and the liver. Hepatology. 1991 May;13(5):977–994. [PubMed] [Google Scholar]
- Pignatelli M., Waters J., Lever A., Iwarson S., Gerety R., Thomas H. C. Cytotoxic T-cell responses to the nucleocapsid proteins of HBV in chronic hepatitis. Evidence that antibody modulation may cause protracted infection. J Hepatol. 1987 Feb;4(1):15–21. doi: 10.1016/s0168-8278(87)80004-1. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Rasokat H., Burger R., Meyer Zum Büschenfelde K. H., Bitter-Suermann D. Quantitative determination of complement components produced by purified hepatocytes. Clin Exp Immunol. 1984 Jan;55(1):189–196. [PMC free article] [PubMed] [Google Scholar]
- Trevisan A., Realdi G., Alberti A., Ongaro G., Pornaro E., Meliconi R. Core antigen-specific immunoglobulin G bound to the liver cell membrane in chronic hepatitis B. Gastroenterology. 1982 Feb;82(2):218–222. [PubMed] [Google Scholar]


