Abstract
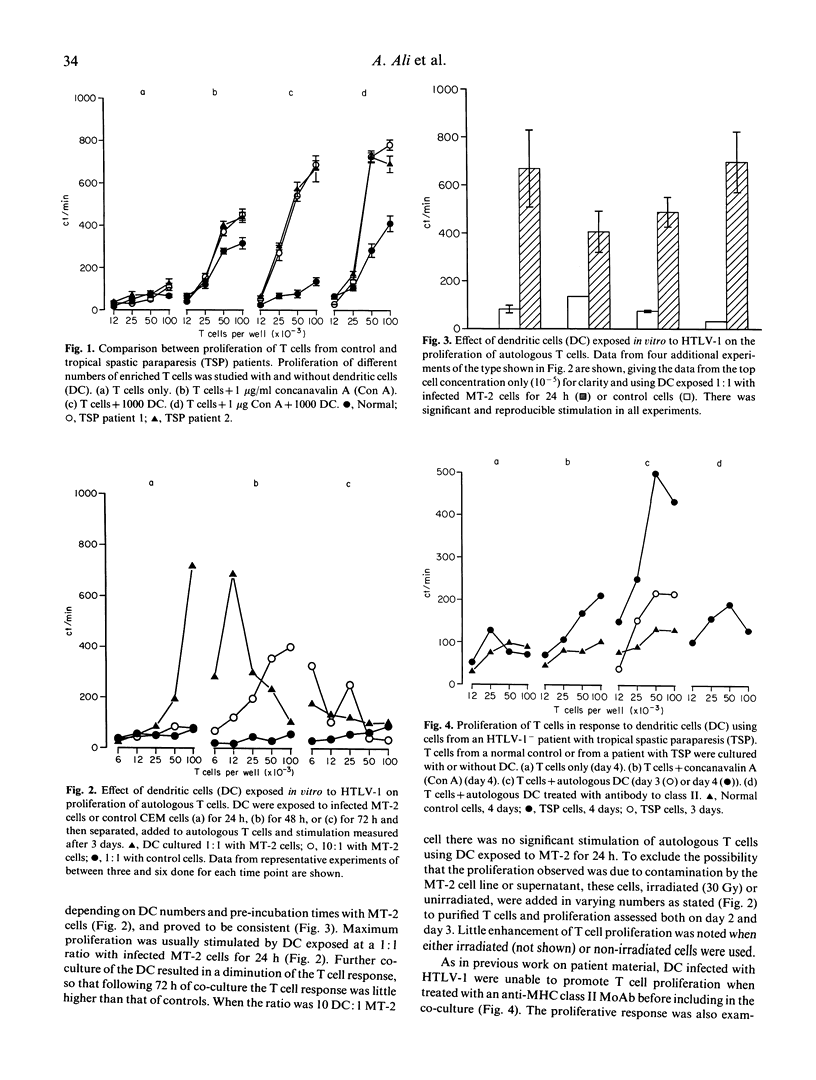
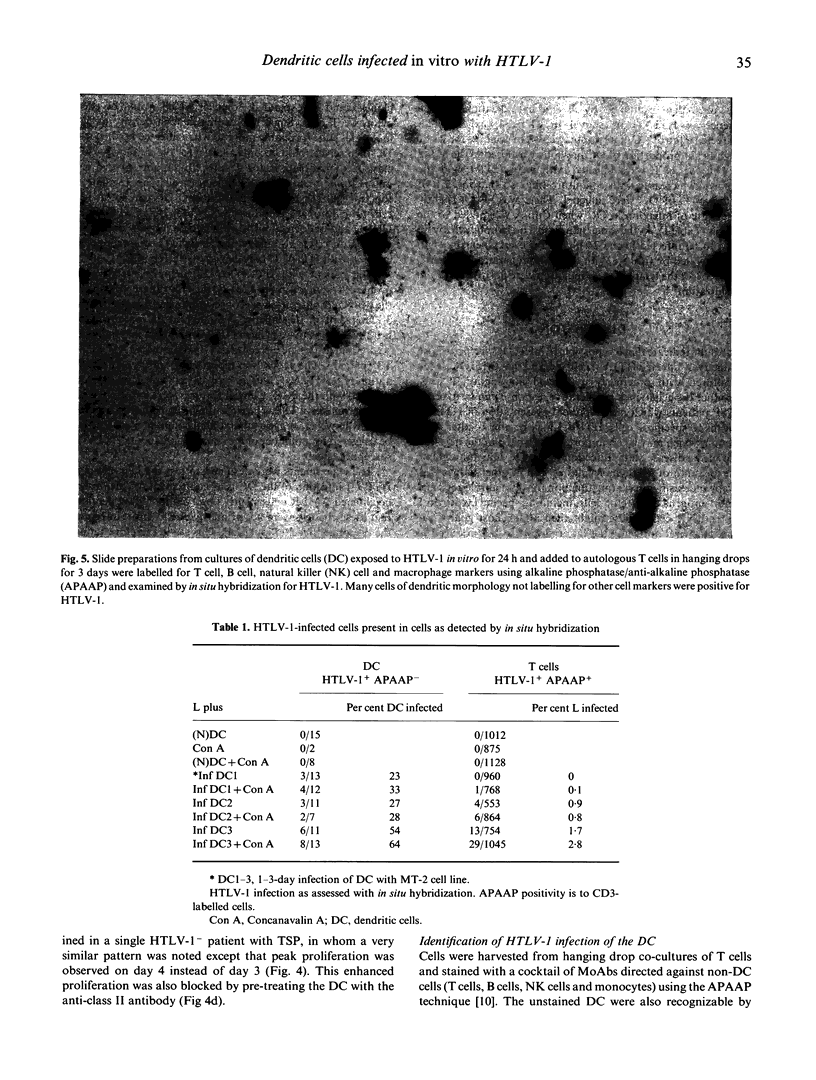
Evidence supporting a role of the dendritic cell (DC) in stimulating autologous T cell activity in tropical spastic paraparesis (TSP) was sought by studies of cells taken from healthy volunteers and exposed to HTLV-1 in vitro. DC were co-cultured with an HTLV-1-producing cell line (MT-2) at 1:1 or 10:1 ratios. These DC stimulated high levels of proliferation in autologous T cells. This was similar to that seen in an autologous mixed leucocyte reaction (AMLR) using cells from TSP patients. The requirement for both DC and virus was confirmed, since neither DC co-cultured with uninfected MT-2 cells nor addition of infected MT-2 cells directly to T cells caused significant stimulation. DC exposed to the highest dose of HTLV-1 (1:1) for 24 h before addition of T cells caused strong stimulation that increased after 8 h but almost disappeared by 72 h. In situ hybridization showed that approximately 25% of DC became infected in cultured cells after preincubation for 24 h, and over 50% were infected with a 72-h preincubation. We suggest that infection of DC by HTLV-1 may be an initial step in altering the immune system in seronegative patients, and that persistent T cell stimulation in those with genetic susceptibility may underlie the production of neurological disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cruickshank J. K., Rudge P., Dalgleish A. G., Newton M., McLean B. N., Barnard R. O., Kendall B. E., Miller D. H. Tropical spastic paraparesis and human T cell lymphotropic virus type 1 in the United Kingdom. Brain. 1989 Aug;112(Pt 4):1057–1090. doi: 10.1093/brain/112.4.1057. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G. The immunology of retrovirus disease. Baillieres Clin Neurol. 1992 Apr;1(1):23–40. [PubMed] [Google Scholar]
- Dalgleish A., Richardson J., Matutes E., Cruickshank K., Newell A., Sinclair A., Thorpe R., Brasher M., Weber J., Catovsky D. HTLV-1 infection in tropical spastic paraparesis: lymphocyte culture and serologic response. AIDS Res Hum Retroviruses. 1988 Dec;4(6):475–485. doi: 10.1089/aid.1988.4.475. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama Y., Kira J., Fujii N., Goto I., Yamamoto N. Increases in helper inducer T cells and activated T cells in HTLV-I-associated myelopathy. Ann Neurol. 1989 Aug;26(2):257–262. doi: 10.1002/ana.410260212. [DOI] [PubMed] [Google Scholar]
- King P. D., Katz D. R. Mechanisms of dendritic cell function. Immunol Today. 1990 Jun;11(6):206–211. doi: 10.1016/0167-5699(90)90084-m. [DOI] [PubMed] [Google Scholar]
- Knight S. C. The autologous mixed lymphocyte reaction and dendritic cells. Br J Rheumatol. 1987 Apr;26(2):85–88. doi: 10.1093/rheumatology/26.2.85. [DOI] [PubMed] [Google Scholar]
- Lowe J., MacLennan K. A., Powe D. G., Pound J. D., Palmer J. B. Microglial cells in human brain have phenotypic characteristics related to possible function as dendritic antigen presenting cells. J Pathol. 1989 Oct;159(2):143–149. doi: 10.1002/path.1711590209. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Cruickshank J. K., Rudge P., Knight S. C. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Gompels M., Pinching A. J., Patterson S., Knight S. C. Antigen-presentation by macrophages but not by dendritic cells in human immunodeficiency virus (HIV) infection. Immunology. 1992 Apr;75(4):576–581. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Patterson S., Knight S. C. Suppression of immune responses by dendritic cells infected with HIV. Immunology. 1989 Jul;67(3):285–289. [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Gross J., Bedford P., Knight S. C. Morphology and phenotype of dendritic cells from peripheral blood and their productive and non-productive infection with human immunodeficiency virus type 1. Immunology. 1991 Mar;72(3):361–367. [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Gross J., Webster A. D. DNA probes bind non-specifically to eosinophils during in situ hybridization: carbol chromotrope blocks binding to eosinophils but does not inhibit hybridization to specific nucleotide sequences. J Virol Methods. 1989 Feb;23(2):105–109. doi: 10.1016/0166-0934(89)90124-9. [DOI] [PubMed] [Google Scholar]
- Patterson S., Knight S. C. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987 Apr;68(Pt 4):1177–1181. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- Prince H. E., Weber D. M., Jensen E. R. Spontaneous lymphocyte proliferation in HTLV-I/II infection reflects preferential activation of CD8 and CD16/56 cell subsets. Clin Immunol Immunopathol. 1991 Mar;58(3):419–430. doi: 10.1016/0090-1229(91)90132-t. [DOI] [PubMed] [Google Scholar]
- Richardson J. H., Edwards A. J., Cruickshank J. K., Rudge P., Dalgleish A. G. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990 Nov;64(11):5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M., Suzumura A., Yoshida M., Marunouchi T. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J Virol. 1990 Aug;64(8):4002–4006. doi: 10.1128/jvi.64.8.4002-4006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. D., Gilbertson S. M., Rowley D. A. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985 Aug 1;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. D., Keij J., Gilbertson S. M., Rowley D. A. Thy-1+ and Thy-1- natural killer cells. Only Thy-1- natural killer cells suppress dendritic cells. J Exp Med. 1986 Apr 1;163(4):1012–1017. doi: 10.1084/jem.163.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]