Abstract
Subpopulations of human T cells (Th0, Th1 and Th2) can be distinguished by their cytokine-secretion pattern. Evidence is increasing from other studies that the outcome of a human disease may depend on the subpopulation of T cells that predominates at the site of inflammation. Reactive arthritis serves as a useful model of chronic inflammatory diseases, because the triggering antigen can be identified. Using this triggering antigen we raised 33 T cell clones reactive with Chlamydia trachomatis and 25 T cell clones that were not reactive, all from the synovial fluid of two patients suffering from Chlamydia-induced arthritis. Their cytokine secretion patterns for interferon-gamma (IFN-gamma), IL-2 and IL-4 were analysed, as also were mRNAs for IFN-gamma and IL-10 by in situ hybridization. Out of the 33 antigen-reactive clones 23 showed a Th1 pattern with IFN-gamma but not IL-4 secretion, while the remaining 10 exhibited a Th0 pattern. The clones that did not react with Chlamydia expressed all patterns of cytokine secretion, including a Th2 pattern, thus providing a control population that excludes bias in the sampling procedure. CD4 and CD8 clones displayed a similar cytokine-secretion pattern. In addition this study demonstrates for the first time the expression of IL-10 mRNA in T cell clones derived from synovial fluid, and this was not confined to the Th2 subset. The Th1 response that Chlamydia provoke can be regarded as appropriate for such an obligate intracellular pathogen.
Full text
PDF


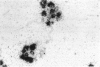
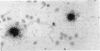
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Modlin R. L., Salgame P. Stigma variations: observations on suppressor T cells and leprosy. Annu Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- Brewerton D. A., Caffrey M., Nicholls A., Walters D., Oates J. K., James D. C. Reiter's disease and HL-A 27. Lancet. 1973 Nov 3;302(7836):996–998. doi: 10.1016/s0140-6736(73)91091-x. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Schobert C. S., Williams D. M., Krueger D. A. Characterization of gamma interferon-mediated cytotoxicity to chlamydia-infected fibroblasts. Infect Immun. 1989 Mar;57(3):870–874. doi: 10.1128/iai.57.3.870-874.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keat A. Reiter's syndrome and reactive arthritis in perspective. N Engl J Med. 1983 Dec 29;309(26):1606–1615. doi: 10.1056/NEJM198312293092604. [DOI] [PubMed] [Google Scholar]
- Keat A., Thomas B., Dixey J., Osborn M., Sonnex C., Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987 Jan 10;1(8524):72–74. doi: 10.1016/s0140-6736(87)91910-6. [DOI] [PubMed] [Google Scholar]
- Lahesmaa R., Yssel H., Batsford S., Luukkainen R., Möttönen T., Steinman L., Peltz G. Yersinia enterocolitica activates a T helper type 1-like T cell subset in reactive arthritis. J Immunol. 1992 May 15;148(10):3079–3085. [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Rahman M. U., Cheema M. A., Schumacher H. R., Hudson A. P. Molecular evidence for the presence of chlamydia in the synovium of patients with Reiter's syndrome. Arthritis Rheum. 1992 May;35(5):521–529. doi: 10.1002/art.1780350506. [DOI] [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Schlaak J., Hermann E., Ringhoffer M., Probst P., Gallati H., Meyer zum Büschenfelde K. H., Fleischer B. Predominance of Th1-type T cells in synovial fluid of patients with Yersinia-induced reactive arthritis. Eur J Immunol. 1992 Nov;22(11):2771–2776. doi: 10.1002/eji.1830221103. [DOI] [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Shemer-Avni Y., Wallach D., Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988 Sep;56(9):2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieper J., Braun J., Brandt J., Miksits K., Heesemann J., Laitko S., Sörensen H., Distler A., Kingsley G. Pathogenetic role of Chlamydia, Yersinia and Borrelia in undifferentiated oligoarthritis. J Rheumatol. 1992 Aug;19(8):1236–1242. [PubMed] [Google Scholar]
- Sieper J., Braun J., Wu P., Kingsley G. Alteration in T cell/macrophage ratio may reveal lymphocyte proliferation specific for the triggering antigen in reactive arthritis. Scand J Immunol. 1992 Sep;36(3):427–434. doi: 10.1111/j.1365-3083.1992.tb02957.x. [DOI] [PubMed] [Google Scholar]
- Sieper J., Braun J., Wu P., Kingsley G. T cells are responsible for the enhanced synovial cellular immune response to triggering antigen in reactive arthritis. Clin Exp Immunol. 1993 Jan;91(1):96–102. doi: 10.1111/j.1365-2249.1993.tb03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieper J., Kingsley G., Palacios-Boix A., Pitzalis C., Treharne J., Hughes R., Keat A., Panayi G. S. Synovial T lymphocyte-specific immune response to Chlamydia trachomatis in Reiter's disease. Arthritis Rheum. 1991 May;34(5):588–598. doi: 10.1002/art.1780340511. [DOI] [PubMed] [Google Scholar]
- Ward M. E. Chlamydial classification, development and structure. Br Med Bull. 1983 Apr;39(2):109–115. doi: 10.1093/oxfordjournals.bmb.a071800. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]
- Yssel H., Shanafelt M. C., Soderberg C., Schneider P. V., Anzola J., Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991 Sep 1;174(3):593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H. Do human TH1 and TH2 CD4+ clones exist? Res Immunol. 1991 Jan;142(1):59–63. doi: 10.1016/0923-2494(91)90014-a. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]