Abstract
The perpetuation of inflammation in ulcerative colitis and Crohn's disease may be regulated in part by an increased secretion of proinflammatory cytokines due to either an appropriate response to initial stimulating agents, and/or due to an impaired down-regulation of cytokine secretion. The aim of this study was to determine the secretion patterns of the proinflammatory cytokines tumour necrosis factor-alpha (TNF-alpha), IL-6 and IL-1 beta, from isolated lamina propria mononuclear cells (LPMNC) isolated from colonic biopsies from patients with untreated ulcerative colitis or Crohn's disease. LPMNC isolated from involved inflammatory bowel disease (IBD) mucosa spontaneously produced increased amounts of TNF-alpha, and IL-6, and IL-1 beta. The TNF-alpha secretion from IBD LPMNC could be further enhanced by pokeweed mitogen stimulation. The secretion patterns of TNF-alpha and IL-1 beta by LPMNC from patients with either ulcerative colitis or Crohn's disease demonstrated a close correlation with the degree of tissue involvement and mucosal inflammation. LPMNC from non-involved ulcerative colitis mucosa secreted markedly increased levels of IL-6 compared with non-involved Crohn's disease mucosa or control mucosa. The heightened IL-6 secretion from LPMNC from non-involved ulcerative colitis mucosa without visible or microscopic signs of inflammation indicates that the pathophysiologic mechanisms involved in the initiation of inflammation may differ between ulcerative colitis and Crohn's disease. The determination of proinflammatory cytokine secretion by isolated LPMNC from colonoscopic biopsies may be a sensitive method for monitoring the severity of mucosal inflammation in IBD patients.
Full text
PDF

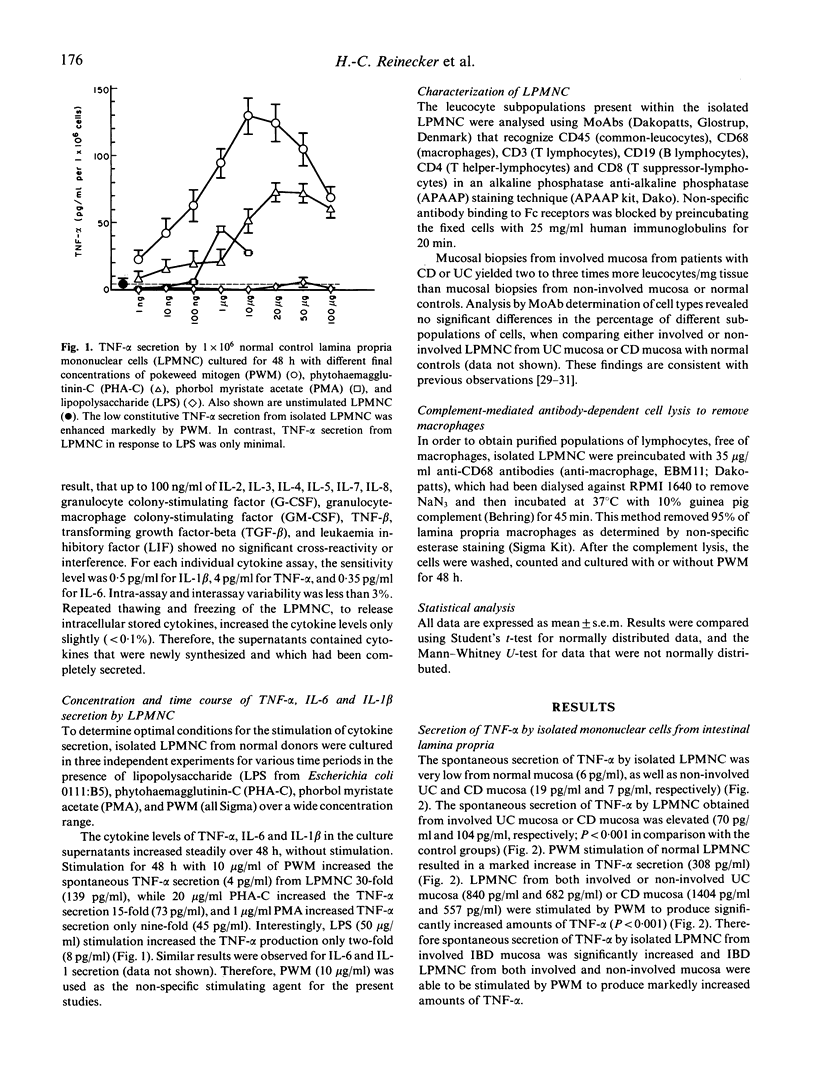
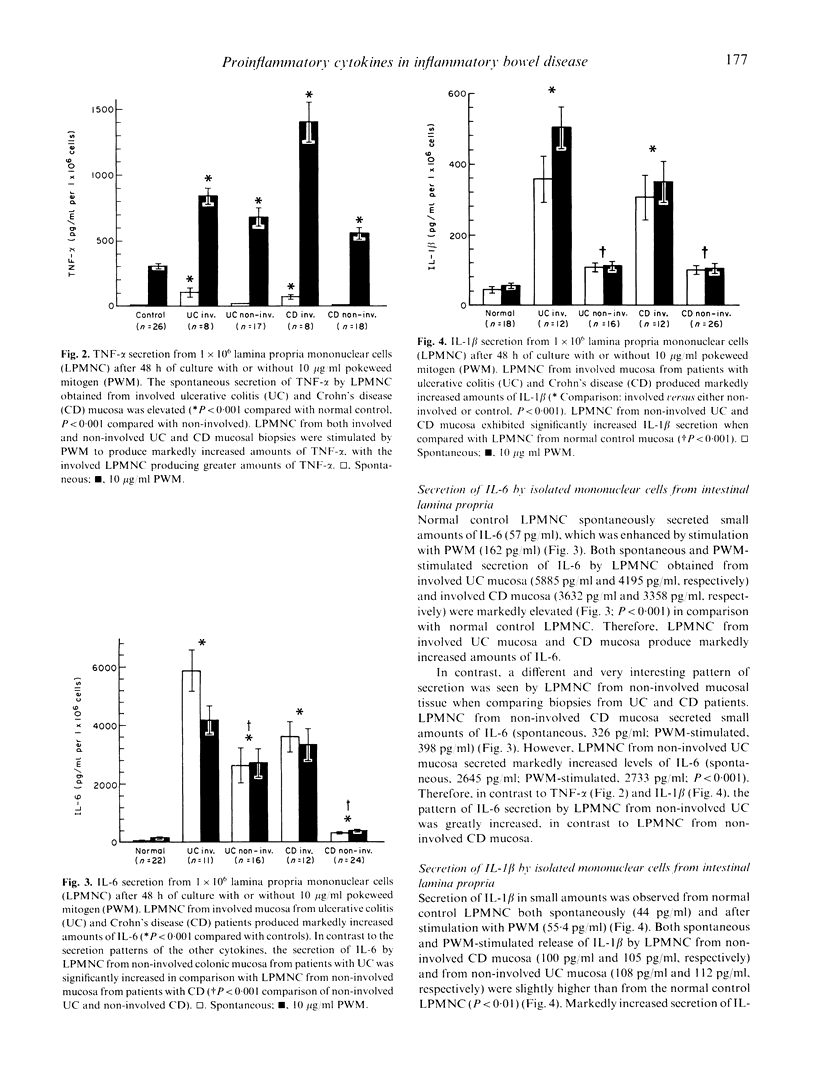




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Gross V., Cäsar I., Krumm D., Hosp J., David M., Schölmerich J. Activation of monocytes during inflammatory bowel disease. Pathobiology. 1991;59(3):166–170. doi: 10.1159/000163637. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Clark B. D., Schindler R., Lierena R., Eysselein V. E., Thompson R. C., Dinarello C. A. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990 Sep;86(3):972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P., Campieri M., Belluzzi A., Boni P., Brignola C., Ferretti H., Iannone P., Miglioli M., Barbara L. Interleukin 1 in ulcerative colitis. Gut. 1991 Mar;32(3):338–338. doi: 10.1136/gut.32.3.338-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross V., Andus T., Caesar I., Roth M., Schölmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992 Feb;102(2):514–519. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- Hilbert D. M., Cancro M. P., Scherle P. A., Nordan R. P., Van Snick J., Gerhard W., Rudikoff S. T cell derived IL-6 is differentially required for antigen-specific antibody secretion by primary and secondary B cells. J Immunol. 1989 Dec 15;143(12):4019–4024. [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Hoffman M., Weinberg J. B. Tumor necrosis factor-alpha induces increased hydrogen peroxide production and Fc receptor expression, but not increased Ia antigen expression by peritoneal macrophages. J Leukoc Biol. 1987 Dec;42(6):704–707. doi: 10.1002/jlb.42.6.704. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyams J. S., Treem W. R., Eddy E., Wyzga N., Moore R. E. Tumor necrosis factor-alpha is not elevated in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1991 Feb;12(2):233–236. doi: 10.1097/00005176-199102000-00016. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Immunoregulatory function of lamina propria T cells in Crohn's disease. Gastroenterology. 1985 May;88(5 Pt 1):1143–1150. doi: 10.1016/s0016-5085(85)80073-1. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986 Dec;91(6):1483–1489. [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Ranyard J., Souza L., Shepard M., Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood. 1987 Jul;70(1):55–59. [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Ziegler-Heitbrock H. W., Mertelsmann R., Herrmann F. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. J Clin Invest. 1989 Apr;83(4):1308–1312. doi: 10.1172/JCI114016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- MacDonald T. T., Hutchings P., Choy M. Y., Murch S., Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990 Aug;81(2):301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Kurlac L., Gallagher A., Hawkey C. J. High circulating concentrations of interleukin-6 in active Crohn's disease but not ulcerative colitis. Gut. 1991 Dec;32(12):1531–1534. doi: 10.1136/gut.32.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Fujihashi K., Beagley K. W., Kiyono H. Role of interleukin-6 in human and mouse mucosal IgA plasma cell responses. Immunol Res. 1991;10(3-4):418–422. doi: 10.1007/BF02919734. [DOI] [PubMed] [Google Scholar]
- Mican J. A., Arora N., Burd P. R., Metcalfe D. D. Passive cutaneous anaphylaxis in mouse skin is associated with local accumulation of interleukin-6 mRNA and immunoreactive interleukin-6 protein. J Allergy Clin Immunol. 1992 Nov;90(5):815–824. doi: 10.1016/0091-6749(92)90107-d. [DOI] [PubMed] [Google Scholar]
- Mitsuyama K., Sata M., Tanikawa K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn. 1991 Feb;26(1):20–28. doi: 10.1007/BF02779504. [DOI] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Murch S. H., Lamkin V. A., Savage M. O., Walker-Smith J. A., MacDonald T. T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991 Aug;32(8):913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T., Mizuta T., Rosén A., Hirano T., Kishimoto T., Honjo T. Enhancement of the interleukin 2 receptor expression on T cells by multiple B-lymphotropic lymphokines. Immunol Lett. 1987 Jul;15(3):249–253. doi: 10.1016/0165-2478(87)90032-0. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Roldán E., Brieva J. A. Terminal differentiation of human bone marrow cells capable of spontaneous and high-rate immunoglobulin secretion: role of bone marrow stromal cells and interleukin 6. Eur J Immunol. 1991 Nov;21(11):2671–2677. doi: 10.1002/eji.1830211105. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Chadwick V. S., Hodgson H. J. Granulocyte migration in ulcerative colitis. Eur J Clin Invest. 1985 Apr;15(2):60–63. doi: 10.1111/j.1365-2362.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Crofton M. E., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. 111Indium autologous granulocytes in the detection of inflammatory bowel disease. Gut. 1985 Sep;26(9):955–960. doi: 10.1136/gut.26.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölmerich J., Schmidt E., Schümichen C., Billmann P., Schmidt H., Gerok W. Scintigraphic assessment of bowel involvement and disease activity in Crohn's disease using technetium 99m-hexamethyl propylene amine oxine as leukocyte label. Gastroenterology. 1988 Nov;95(5):1287–1293. doi: 10.1016/0016-5085(88)90363-0. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen M., Reinecker H. C., Petersen J., Doehn C., Pflüger I., Voss A., Raedler A. Differences in cytokine secretion by intestinal mononuclear cells, peripheral blood monocytes and alveolar macrophages from HIV-infected patients. Clin Exp Immunol. 1993 Jan;91(1):30–36. doi: 10.1111/j.1365-2249.1993.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M. L., Zanker B., Muggia A., Antonioli D., Peppercorn M. A., Strom T. B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992 Jun;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Stringfellow M. M., Wilson R. E., Burakoff S. J., Herrmann S. H. Effect of timing of lymphokine presentation on generation of cytotoxic T lymphocytes. Arch Surg. 1989 Jan;124(1):81–84. doi: 10.1001/archsurg.1989.01410010091019. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Saito H., Kasanuki J., Kishimoto T., Tamura Y., Yoshida S. Significant increase of interleukin 6 production in blood mononuclear leukocytes obtained from patients with active inflammatory bowel disease. Life Sci. 1990;47(24):2193–2197. doi: 10.1016/0024-3205(90)90149-l. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Luce G. E., Webb D., Shaw S. Remote T cell co-stimulation via LFA-1/ICAM-1 and CD2/LFA-3: demonstration with immobilized ligand/mAb and implication in monocyte-mediated co-stimulation. Eur J Immunol. 1991 Jul;21(7):1711–1718. doi: 10.1002/eji.1830210719. [DOI] [PubMed] [Google Scholar]


