Abstract
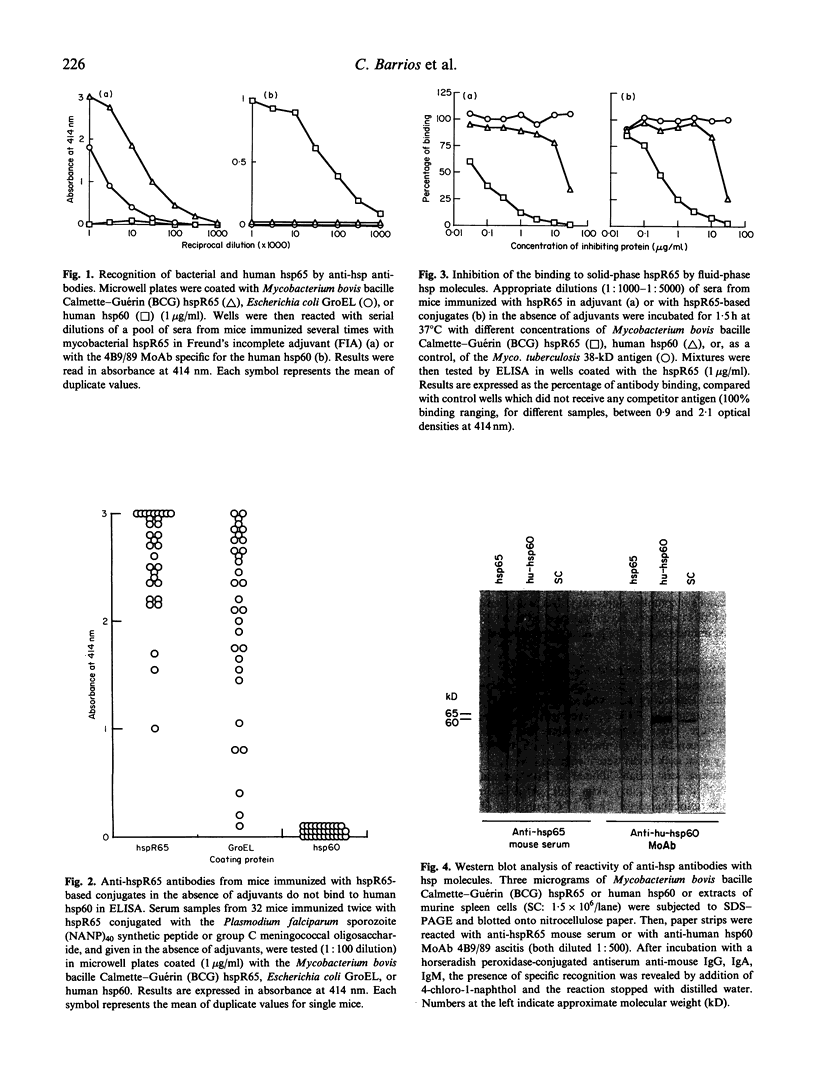
We have previously shown in mice and monkeys that mycobacterial heat shock proteins (hsp) of 65 and 70 kD exert a strong in vivo helper effect when conjugated to synthetic peptides or bacterial oligosaccharides and given in the absence of any adjuvants. Considering the degree of homology existing in the phylogeny among hsp belonging to the same family, we studied whether antibodies induced in mice with this protocol of immunization with the mycobacterial 65-kD hsp (hsp65) would cross-react, and to what extent, with hsp homologues from other origins, notably with the Escherichia coli GroEL protein and with the human homologue (hsp60). The results obtained show that antibodies to the mycobacterial hsp65 cross-reacted with the E. coli GroEL protein, both in ELISA and Western blot experiments, but not with the human hsp60. In competitive ELISA experiments, the binding of these antibodies to solid-phase hsp65 was very effectively inhibited by low concentrations of the mycobacterial hsp65; however, for human hsp60, 100 times higher concentrations were required in order to obtain similar patterns of inhibition. Finally, murine antibodies to the mycobacterial hsp65 always failed to give positive results in Western blot experiments using extracts of murine cells. Taken together, these data suggest that, after immunization of mice with the mycobacterial hsp65 conjugated to peptides or oligosaccharides in the absence of adjuvants, anti-hsp65 antibodies are induced which cross-react well with hsp homologues from other prokaryotes (e.g. E. coli GroEL), but which weakly bind the human hsp homologue. These results may have implications for the potential use of microbial hsp molecules in the design of conjugated vaccine constructs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrios C., Lussow A. R., Van Embden J., Van der Zee R., Rappuoli R., Costantino P., Louis J. A., Lambert P. H., Del Giudice G. Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guérin priming. Eur J Immunol. 1992 Jun;22(6):1365–1372. doi: 10.1002/eji.1830220606. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol. 1991;9:567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Gervaix A., Costantino P., Wyler C. A., Tougne C., de Graeff-Meeder E. R., van Embden J., van der Zee R., Nencioni L., Rappuoli R. Priming to heat shock proteins in infants vaccinated against pertussis. J Immunol. 1993 Mar 1;150(5):2025–2032. [PubMed] [Google Scholar]
- Del Giudice G., Lussow A. R., Lambert P. H. Heat shock proteins as "super"-carriers for sporozoite peptide vaccines? Res Immunol. 1991 Oct;142(8):703–707. doi: 10.1016/0923-2494(91)90153-a. [DOI] [PubMed] [Google Scholar]
- Del Giudice G. New carriers and adjuvants in the development of vaccines. Curr Opin Immunol. 1992 Aug;4(4):454–459. doi: 10.1016/s0952-7915(06)80038-5. [DOI] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Reshef T., Birk O. S., van der Zee R., Walker M. D., Cohen I. R. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaghier A., Prantera C., Bothamley G., Wilkins E., Jindal S., Ivanyi J. Disease association of antibodies to human and mycobacterial hsp70 and hsp60 stress proteins. Clin Exp Immunol. 1992 Aug;89(2):305–309. doi: 10.1111/j.1365-2249.1992.tb06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. B., Coulson A. F., Duff G. W. Sequence homologies between hsp60 and autoantigens. Immunol Today. 1993 Mar;14(3):115–118. doi: 10.1016/0167-5699(93)90210-C. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lussow A. R., Aguado M. T., Del Giudice G., Lambert P. H. Towards vaccine optimisation. Immunol Lett. 1990 Aug;25(1-3):255–263. doi: 10.1016/0165-2478(90)90124-9. [DOI] [PubMed] [Google Scholar]
- Lussow A. R., Barrios C., van Embden J., Van der Zee R., Verdini A. S., Pessi A., Louis J. A., Lambert P. H., Del Giudice G. Mycobacterial heat-shock proteins as carrier molecules. Eur J Immunol. 1991 Oct;21(10):2297–2302. doi: 10.1002/eji.1830211002. [DOI] [PubMed] [Google Scholar]
- Perraut R., Lussow A. R., Gavoille S., Garraud O., Matile H., Tougne C., van Embden J., van der Zee R., Lambert P. H., Gysin J. Successful primate immunization with peptides conjugated to purified protein derivative or mycobacterial heat shock proteins in the absence of adjuvants. Clin Exp Immunol. 1993 Sep;93(3):382–386. doi: 10.1111/j.1365-2249.1993.tb08189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. Malaria vaccines. Curr Opin Immunol. 1992 Aug;4(4):432–441. doi: 10.1016/s0952-7915(06)80035-x. [DOI] [PubMed] [Google Scholar]
- Sharif M., Worrall J. G., Singh B., Gupta R. S., Lydyard P. M., Lambert C., McCulloch J., Rook G. A. The development of monoclonal antibodies to the human mitochondrial 60-kd heat-shock protein, and their use in studying the expression of the protein in rheumatoid arthritis. Arthritis Rheum. 1992 Dec;35(12):1427–1433. doi: 10.1002/art.1780351205. [DOI] [PubMed] [Google Scholar]
- Singh B., Gupta R. S. Expression of human 60-kD heat shock protein (HSP60 or P1) in Escherichia coli and the development and characterization of corresponding monoclonal antibodies. DNA Cell Biol. 1992 Jul-Aug;11(6):489–496. doi: 10.1089/dna.1992.11.489. [DOI] [PubMed] [Google Scholar]
- Singh M., Andersen A. B., McCarthy J. E., Rohde M., Schütte H., Sanders E., Timmis K. N. The Mycobacterium tuberculosis 38-kDa antigen: overproduction in Escherichia coli, purification and characterization. Gene. 1992 Aug 1;117(1):53–60. doi: 10.1016/0378-1119(92)90489-c. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venner T. J., Gupta R. S. Nucleotide sequence of mouse HSP60 (chaperonin, GroEL homolog) cDNA. Biochim Biophys Acta. 1990 Nov 30;1087(3):336–338. doi: 10.1016/0167-4781(90)90008-p. [DOI] [PubMed] [Google Scholar]
- Ward J. Prevention of invasive Haemophilus influenzae type b disease: lessons from vaccine efficacy trials. Vaccine. 1991 Jun;9 (Suppl):S17–S25. doi: 10.1016/0264-410x(91)90175-6. [DOI] [PubMed] [Google Scholar]
- Yang X. D., Gasser J., Feige U. Prevention of adjuvant arthritis in rats by a nonapeptide from the 65-kD mycobacterial heat-shock protein. Clin Exp Immunol. 1990 Aug;81(2):189–194. doi: 10.1111/j.1365-2249.1990.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J., Fayet O., Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]