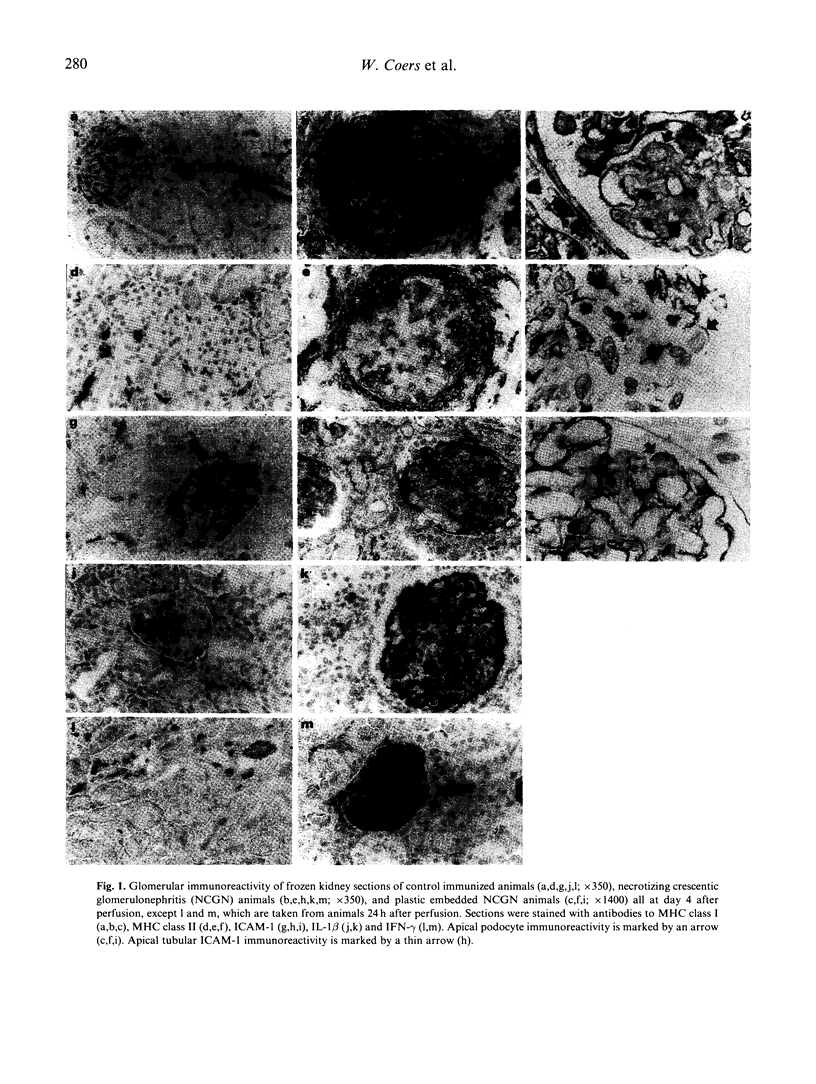
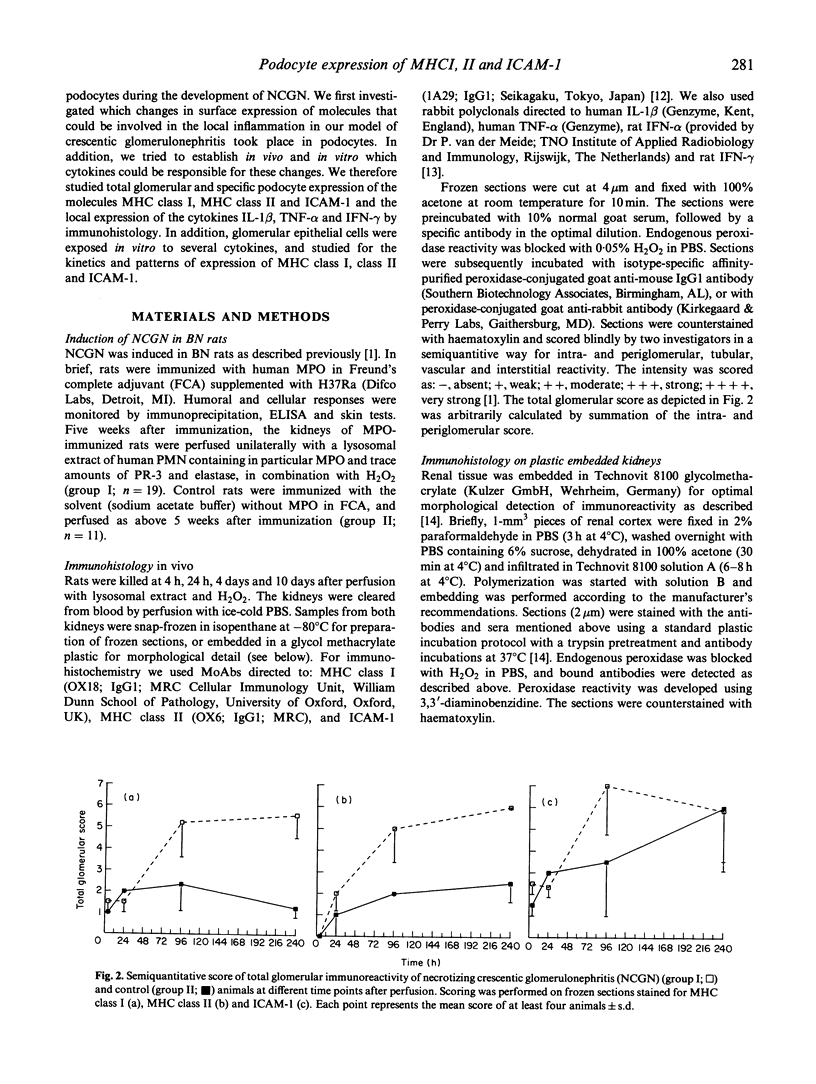
Abstract
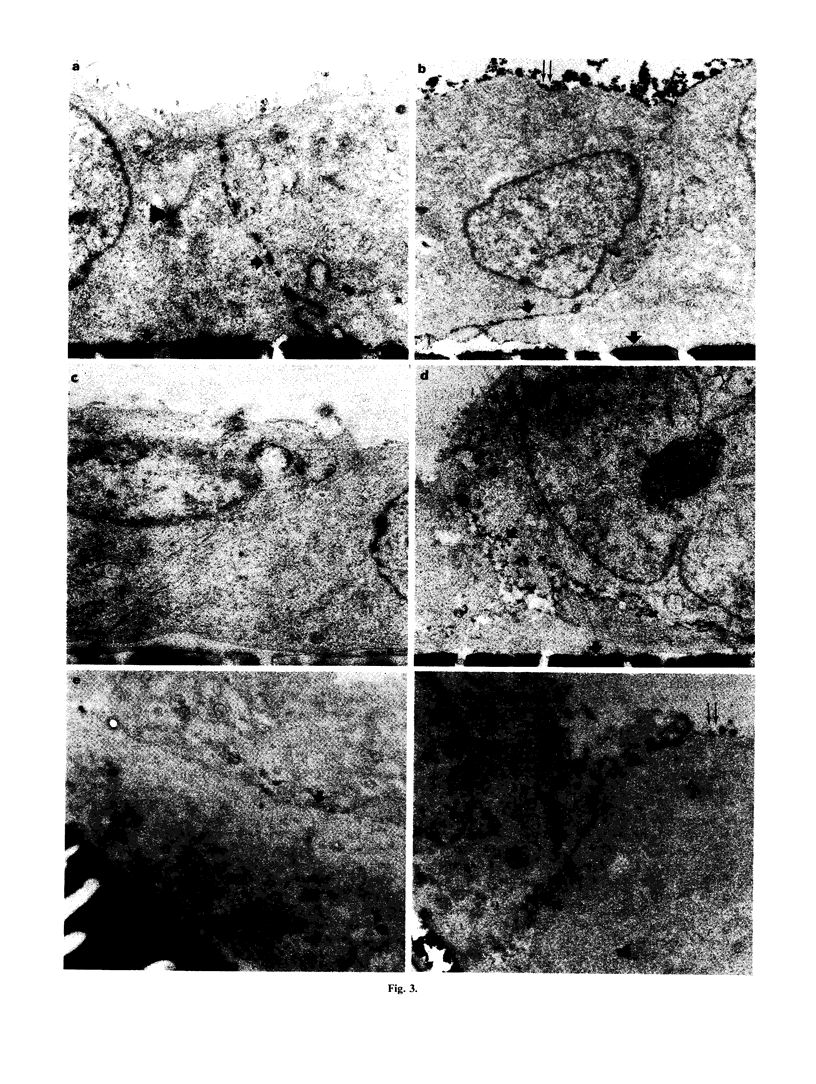
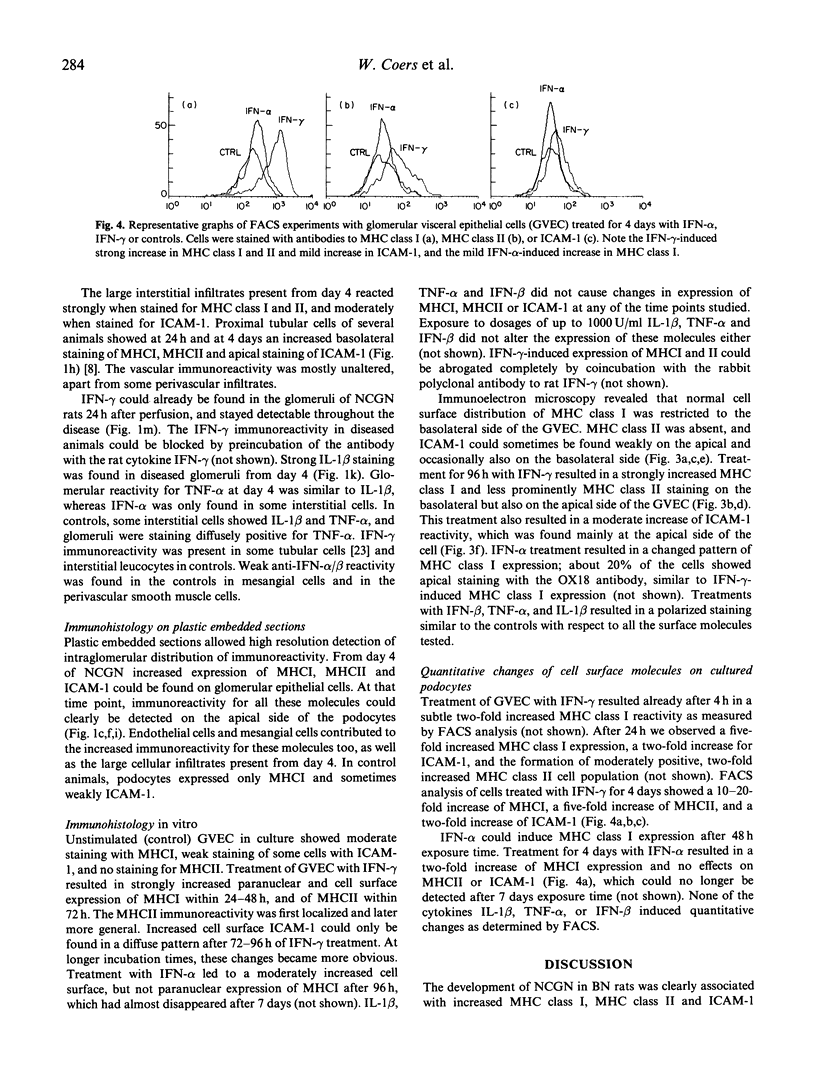
We examined immunopathological changes of podocytes in vivo which, based on in vitro studies, are thought to be relevant for the pathogenesis of renal diseases. We investigated the alterations of podocytes in local inflammation in a recently developed model of pauci-immune necrotizing crescentic glomerulonephritis (NCGN) in the rat. Frozen and plastic embedded kidney sections at different time points of the disease were incubated with antibodies directed to MHC class I, MHC class II, ICAM-1 and to relevant cytokines. Strong glomerular expression of MHC class I, II and ICAM-1 was found within 4 days, and plastic embedded sections clearly demonstrated increased cell membrane staining of podocytes. Increased glomerular interferon-gamma (IFN-gamma) was detected within 24 h of induction of NCGN, and IL-1 beta and tumour necrosis factor-alpha (TNF-alpha) were found from day 4. The potency of these cytokines to induce adhesion molecules on podocytes was investigated on rat glomerular epithelial cells in vitro. By using FACS analysis and electron microscopical techniques, we found that the in vivo expression of MHC class I, II and ICAM-1 by podocytes could in vitro be simulated by IFN-gamma. IFN-alpha weakly induced MHC class I, while IL-1 beta and TNF-alpha were ineffective. We hypothesize that podocytes in this in vivo model are important to maintain the local inflammatory process in the glomerulus by expression of relevant adhesion molecules and MHC molecules upon stimulation with specific cytokines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop G. A., Hall B. M. Expression of leucocyte and lymphocyte adhesion molecules in the human kidney. Kidney Int. 1989 Dec;36(6):1078–1085. doi: 10.1038/ki.1989.303. [DOI] [PubMed] [Google Scholar]
- Brouwer E., Huitema M. G., Klok P. A., de Weerd H., Tervaert J. W., Weening J. J., Kallenberg C. G. Antimyeloperoxidase-associated proliferative glomerulonephritis: an animal model. J Exp Med. 1993 Apr 1;177(4):905–914. doi: 10.1084/jem.177.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers W., Huitema S., Smeenk R. J., Salant D. J., Grond J., Weening J. J. Quantification of glomerular epithelial cell adhesion by using anti-DNA antibodies in ELISA. Hybridoma. 1992 Aug;11(4):529–537. doi: 10.1089/hyb.1992.11.529. [DOI] [PubMed] [Google Scholar]
- Cybulsky A. V., Carbonetto S., Huang Q., McTavish A. J., Cyr M. D. Adhesion of rat glomerular epithelial cells to extracellular matrices: role of beta 1 integrins. Kidney Int. 1992 Nov;42(5):1099–1106. doi: 10.1038/ki.1992.393. [DOI] [PubMed] [Google Scholar]
- Gómez-Chiarri M., Hamilton T. A., Egido J., Emancipator S. N. Expression of IP-10, a lipopolysaccharide- and interferon-gamma-inducible protein, in murine mesangial cells in culture. Am J Pathol. 1993 Feb;142(2):433–439. [PMC free article] [PubMed] [Google Scholar]
- Halloran P. F., Urmson J., Van der Meide P. H., Autenried P. Regulation of MHC expression in vivo. II. IFN-alpha/beta inducers and recombinant IFN-alpha modulate MHC antigen expression in mouse tissues. J Immunol. 1989 Jun 15;142(12):4241–4247. [PubMed] [Google Scholar]
- Kakizaki Y., Kraft N., Atkins R. C. Interferon-gamma stimulates the secretion of IL-1, but not of IL-6, by glomerular mesangial cells. Clin Exp Immunol. 1993 Mar;91(3):521–525. doi: 10.1111/j.1365-2249.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Liu Z. Z., Kashihara N., Wallner E. I. Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol. 1991 Jul;11(4):390–413. [PubMed] [Google Scholar]
- Kawasaki K., Yaoita E., Yamamoto T., Tamatani T., Miyasaka M., Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993 Feb 1;150(3):1074–1083. [PubMed] [Google Scholar]
- Kelley V. R., Singer G. G. The antigen presentation function of renal tubular epithelial cells. Exp Nephrol. 1993 Mar-Apr;1(2):102–111. [PubMed] [Google Scholar]
- Knop J. Immunologic effects of interferon. J Invest Dermatol. 1990 Dec;95(6 Suppl):72S–74S. doi: 10.1111/1523-1747.ep12874780. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Nikolic-Paterson D. J., Zarama M., Vannice J. L., Atkins R. C. Suppression of experimental crescentic glomerulonephritis by the interleukin-1 receptor antagonist. Kidney Int. 1993 Feb;43(2):479–485. doi: 10.1038/ki.1993.70. [DOI] [PubMed] [Google Scholar]
- Madrenas J., Parfrey N. A., Halloran P. F. Interferon gamma-mediated renal MHC expression in mercuric chloride-induced glomerulonephritis. Kidney Int. 1991 Feb;39(2):273–281. doi: 10.1038/ki.1991.33. [DOI] [PubMed] [Google Scholar]
- Maguire J. E., Gresser I., Williams A. H., Kielpinski G. L., Colvin R. B. Modulation of expression of MHC antigens in the kidneys of mice by murine interferon-alpha/beta. Transplantation. 1990 Jan;49(1):130–134. doi: 10.1097/00007890-199001000-00029. [DOI] [PubMed] [Google Scholar]
- Mattila P. M., Nietosvaara Y. A., Ustinov J. K., Renkonen R. L., Häyry P. J. Antigen expression in different parenchymal cell types of rat kidney and heart. Kidney Int. 1989 Aug;36(2):228–233. doi: 10.1038/ki.1989.184. [DOI] [PubMed] [Google Scholar]
- Mendrick D. L., Kelly D. M., Rennke H. G. Antigen processing and presentation by glomerular visceral epithelium in vitro. Kidney Int. 1991 Jan;39(1):71–78. doi: 10.1038/ki.1991.9. [DOI] [PubMed] [Google Scholar]
- Noronha I. L., Krüger C., Andrassy K., Ritz E., Waldherr R. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int. 1993 Mar;43(3):682–692. doi: 10.1038/ki.1993.98. [DOI] [PubMed] [Google Scholar]
- O'Meara Y. M., Natori Y., Minto A. W., Goldstein D. J., Manning E. C., Salant D. J. Nephrotoxic antiserum identifies a beta 1-integrin on rat glomerular epithelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):F1083–F1091. doi: 10.1152/ajprenal.1992.262.6.F1083. [DOI] [PubMed] [Google Scholar]
- Quigg R. J., Cybulsky A. V., Jacobs J. B., Salant D. J. Anti-Fx1A produces complement-dependent cytotoxicity of glomerular epithelial cells. Kidney Int. 1988 Jul;34(1):43–52. doi: 10.1038/ki.1988.143. [DOI] [PubMed] [Google Scholar]
- Reivinen J., Holthöfer H., Miettinen A. A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int. 1992 Sep;42(3):624–631. doi: 10.1038/ki.1992.327. [DOI] [PubMed] [Google Scholar]
- Sacks S. H., Zhou W., Pani A., Campbell R. D., Martin J. Complement C3 gene expression and regulation in human glomerular epithelial cells. Immunology. 1993 Jul;79(3):348–354. [PMC free article] [PubMed] [Google Scholar]
- Schmouder R. L., Strieter R. M., Kunkel S. L. Interferon-gamma regulation of human renal cortical epithelial cell-derived monocyte chemotactic peptide-1. Kidney Int. 1993 Jul;44(1):43–49. doi: 10.1038/ki.1993.211. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Waldherr R., Noronha I. L., Niemir Z., Krüger C., Stein H., Stumm G. Expression of cytokines and growth factors in human glomerulonephritides. Pediatr Nephrol. 1993 Aug;7(4):471–478. doi: 10.1007/BF00857578. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Nakagawa H. Cytokines enhance the production of a chemotactic factor for polymorphonuclear leukocytes by rat renal glomerular epithelioid cells. Nephron. 1990;54(2):169–175. doi: 10.1159/000185839. [DOI] [PubMed] [Google Scholar]
- Whicher J. T., Evans S. W. Cytokines in disease. Clin Chem. 1990 Jul;36(7):1269–1281. [PubMed] [Google Scholar]
- Wuthrich R. P., Glimcher L. H., Yui M. A., Jevnikar A. M., Dumas S. E., Kelley V. E. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990 Feb;37(2):783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Takaeda M., Wada T., Ogi M., Tomosugi N., Takabatake T., Abe T., Yoshimura M., Kida H., Kobayashi K. Intraglomerular expression of MHC class II and Ki-67 antigens and serum gamma-interferon levels in IgA nephropathy. Nephron. 1992;62(2):169–175. doi: 10.1159/000187028. [DOI] [PubMed] [Google Scholar]
- van Goor H., van der Horst M. L., Fidler V., Grond J. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992 May;66(5):564–571. [PubMed] [Google Scholar]
- van der Meide P. H., Borman A. H., Beljaars H. G., Dubbeld M. A., Botman C. A., Schellekens H. Isolation and characterization of monoclonal antibodies directed to rat interferon-gamma. Lymphokine Res. 1989 Winter;8(4):439–449. [PubMed] [Google Scholar]