Abstract
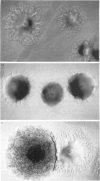
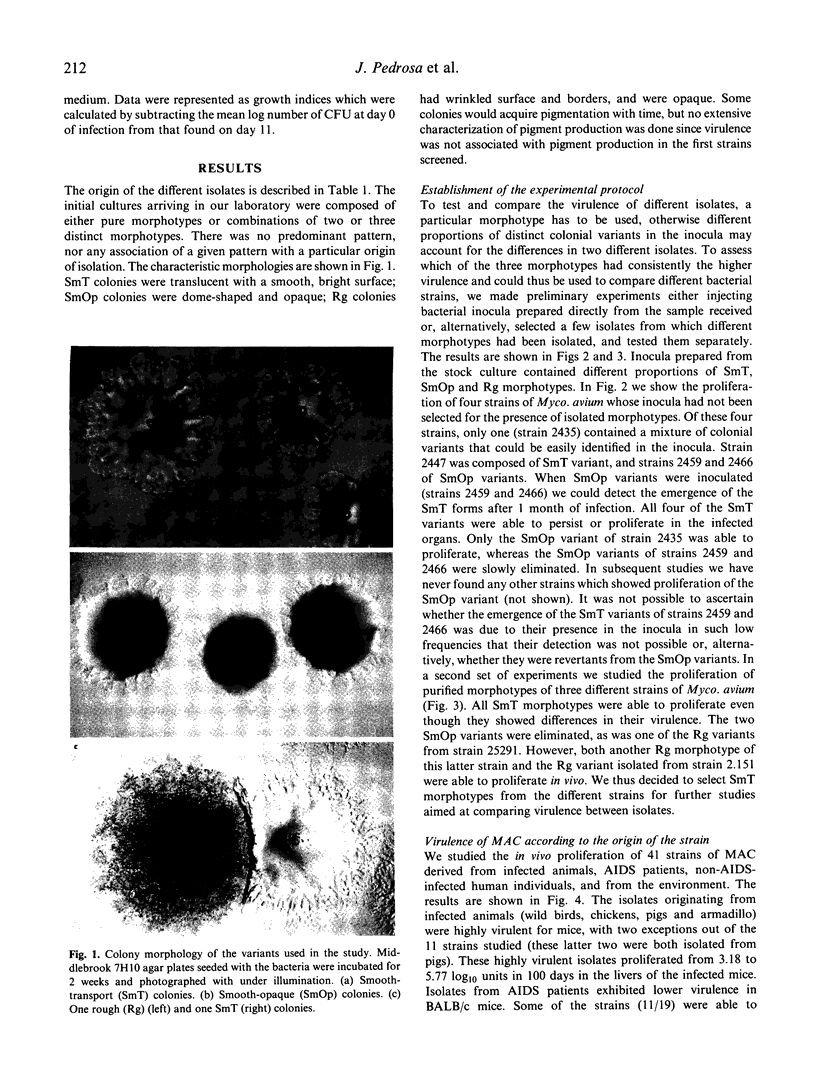
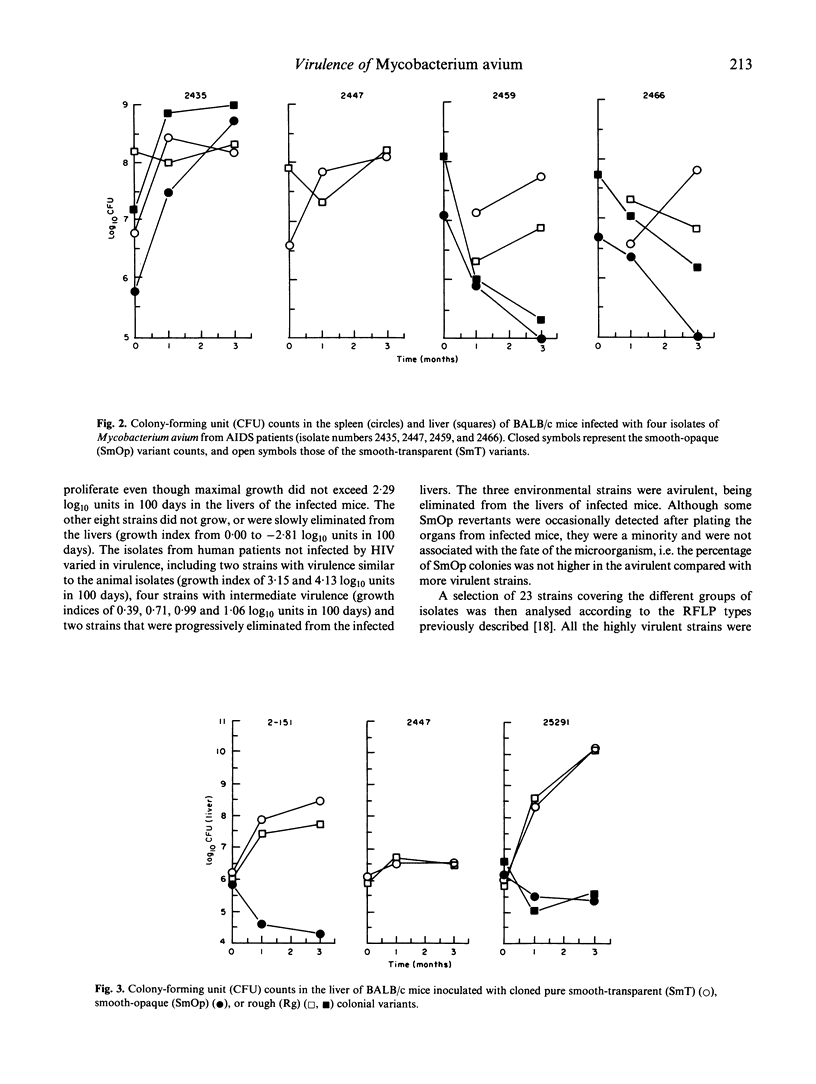
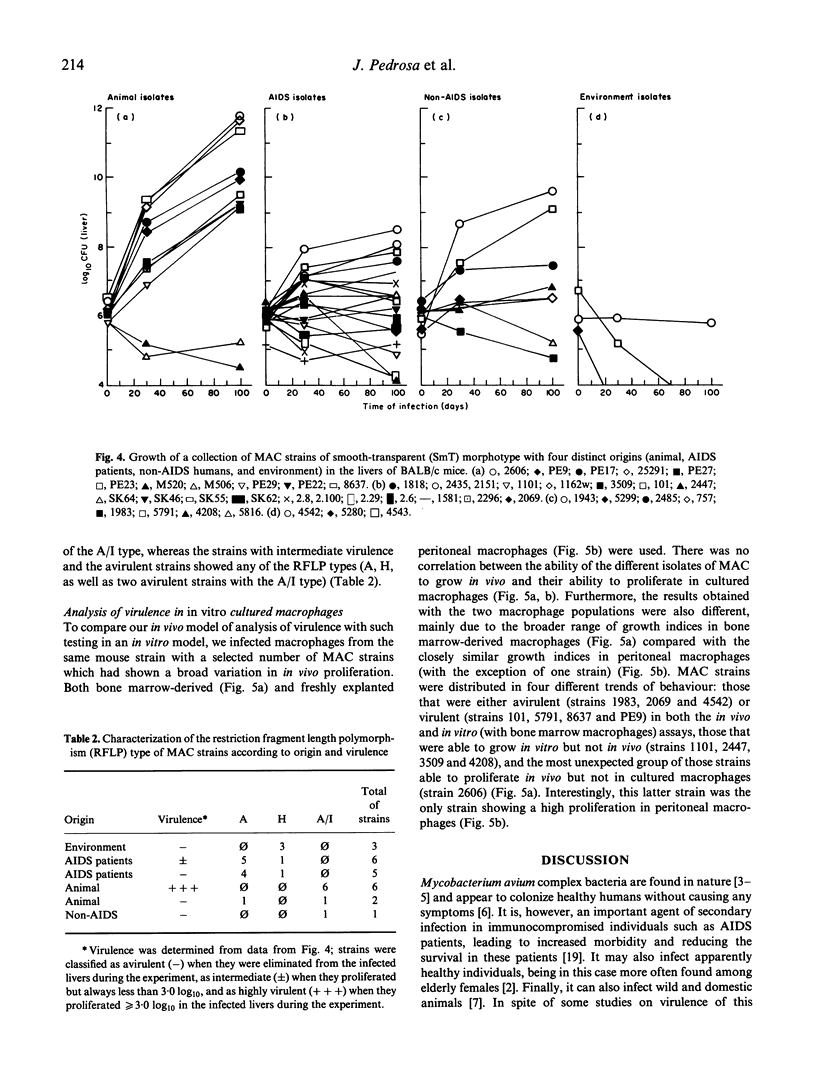
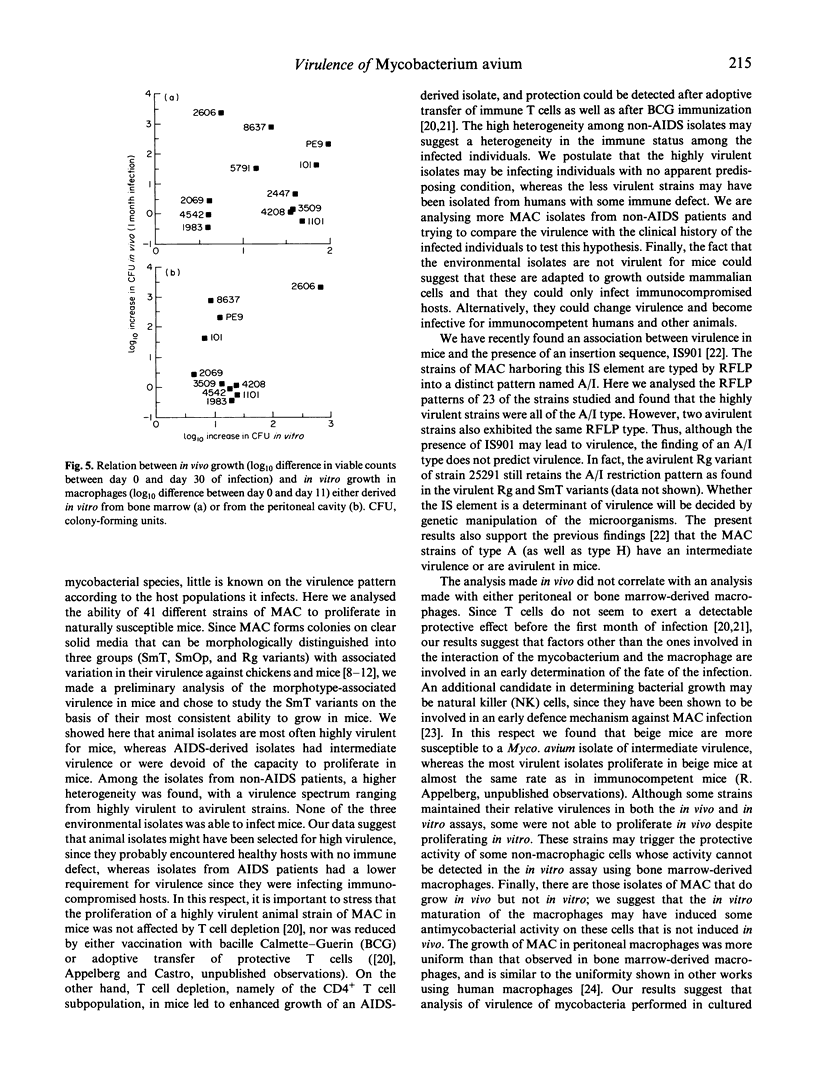
The virulence of different isolates of MAC was studied in naturally susceptible BALB/c mice. In preliminary experiments, MAC bacteria forming smooth transparent colonies on solid media (SmT variants) were found to be virulent for BALB/c mice, causing progressive infection; smooth opaque (SmOp) were generally avirulent, being slowly eliminated from the infected organs; and rough (Rg) variants were either avirulent or as virulent as SmT variants. We chose to compare the virulence of different isolates of MAC of different origins, studying only the SmT morphotype. Strains of MAC isolated from naturally infected animals were those that most consistently caused progressive infections. AIDS patients-derived isolates were of intermediate virulence or devoid of virulence in mice. The environmental strains were eliminated from mice or did not proliferate. Strains of MAC isolated from individuals who were not infected by HIV varied in virulence from completely avirulent to highly virulent. There was no close correlation between virulence and restriction fragment length polymorphism (RFLP) type, although all highly virulent strains were of the A/I type. There was also no correlation between virulence analysed in vivo and the ability to grow in cultured macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Castro A. G., Pedrosa J., Silva R. A., Orme I. M., Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994 Sep;62(9):3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Orme I. M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993 Nov;80(3):352–359. [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Orme I. M., Pinto de Sousa M. I., Silva M. T. In vitro effects of interleukin-4 on interferon-gamma-induced macrophage activation. Immunology. 1992 Aug;76(4):553–559. [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin Exp Immunol. 1992 Mar;87(3):379–385. doi: 10.1111/j.1365-2249.1992.tb03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. W., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. V. Numbers in eastern United States soils and correlation with soil characteristics. Am Rev Respir Dis. 1984 Oct;130(4):630–633. doi: 10.1164/arrd.1984.130.4.630. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Parker B. C., Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis. 1980 Jun;121(6):931–937. doi: 10.1164/arrd.1980.121.6.931. [DOI] [PubMed] [Google Scholar]
- Harshan K. V., Gangadharam P. R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991 Aug;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Havlik J. A., Ellis D. A., Kennedy E., Fann S. A., Dubois R. E., Thompson S. E. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):557–559. doi: 10.1164/ajrccm/144.3_Pt_1.557. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Kunze Z. M., Wall S., Appelberg R., Silva M. T., Portaels F., McFadden J. J. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol Microbiol. 1991 Sep;5(9):2265–2272. doi: 10.1111/j.1365-2958.1991.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Lévy-Frébault V. V., Portaels F. Proposed minimal standards for the genus Mycobacterium and for description of new slowly growing Mycobacterium species. Int J Syst Bacteriol. 1992 Apr;42(2):315–323. doi: 10.1099/00207713-42-2-315. [DOI] [PubMed] [Google Scholar]
- McFadden J. J., Kunze Z. M., Portaels F., Labrousse V., Rastogi N. Epidemiological and genetic markers, virulence factors and intracellular growth of Mycobacterium avium in AIDS. Res Microbiol. 1992 May;143(4):423-30, discussion 430-6. doi: 10.1016/0923-2508(92)90057-u. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Solotorovsky M. R. Relationship of colonial morphology to virulence for chickens of Mycobacterium avium and the nonphotochromogens. Am Rev Respir Dis. 1965 Nov;92(5):704–713. doi: 10.1164/arrd.1965.92.5.704. [DOI] [PubMed] [Google Scholar]
- Pattyn S. R., Hermans-Boveroulle M. T. Dissociation in M. avium. Pneumonologie. 1970;142(2):119–125. doi: 10.1007/BF02095206. [DOI] [PubMed] [Google Scholar]
- Portaels F., Asselineau C., Baess I., Daffé M., Dobson G., Draper P., Gregory D., Hall R. M., Imaeda T., Jenkins P. A. A cooperative taxonomic study of mycobacteria isolated from armadillos infected with Mycobacterium leprae. J Gen Microbiol. 1986 Oct;132(10):2693–2707. doi: 10.1099/00221287-132-10-2693. [DOI] [PubMed] [Google Scholar]
- Portaels F., Larsson L., Smeets P. Isolation of mycobacteria from healthy persons' stools. Int J Lepr Other Mycobact Dis. 1988 Sep;56(3):468–471. [PubMed] [Google Scholar]
- Prince D. S., Peterson D. D., Steiner R. M., Gottlieb J. E., Scott R., Israel H. L., Figueroa W. G., Fish J. E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989 Sep 28;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Toba H., Crawford J. T., Ellner J. J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989 Jan;57(1):239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt S. L., George K. L., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous Mycobacteria. III. Isolation of potentially pathogenic mycobacteria from aerosols. Am Rev Respir Dis. 1980 Aug;122(2):259–263. doi: 10.1164/arrd.1980.122.2.259. [DOI] [PubMed] [Google Scholar]