Abstract
The central role of type-2 helper T (Th2) cells in the development of allergic responses and immune responses against helminthic parasites is well documented. The differentiation of Th2 cells from naive T cells requires both the recognition of antigen by T cell antigen receptors (TCR) and the activation of downstream signal-transduction molecules of the interleukin 4 receptor (IL-4R) pathway, including Jak1, Jak3, and STAT6. Little is known, however, about how these two distinct pathways cooperate with each other to induce Th2 cells. Here, we use a T cell-specific H-Ras-dominant-negative transgenic mouse to show that TCR-mediated activation of the Ras/mitogen-activated protein kinase pathway alters IL-4R function and is required for Th2 cell differentiation. The enhancement of IL-4R signaling seems to be a consequence of both direct “crosstalk” with the TCR signaling pathway and increased protein expression of downstream signaling molecules of the IL-4R pathway. Therefore, successful Th2 differentiation depends on the effectiveness of the TCR-mediated activation of the Ras/mitogen-activated protein kinase pathway in modifying the IL-4R-mediated signaling pathway.
CD4+ helper T cells can be divided into two distinct subpopulations: type-1 helper T (Th1) cells and type-2 helper T (Th2) cells (1). Th1 cells produce interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor β, whereas Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13. The development of Th1 and Th2 cells is central to the diversity of helper T cell-dependent immune responses in infectious, allergic, and autoimmune diseases (2–4). Th1 cells mediate delayed type hypersensitivity and organ-specific autoimmune diseases, whereas Th2 cells play important roles in allergic and infectious diseases.
Both Th1 and Th2 cells differentiate from a common precursor, and the direction of differentiation into Th1 and Th2 cells depends on the exogenous cytokines present during primary antigenic stimulation of naive T cells (2–4). IL-12 promotes the differentiation of naive T cells into Th1 effector cells (5, 6), whereas IL-4 is required for Th2 cell differentiation (7, 8). For Th2 cell differentiation, the activation of signal-transduction pathways downstream of the IL-4 receptor (IL-4R) is essential, as evidenced by the fact that mice harboring gene disruptions for IL-4 (9, 10) or STAT6 (11–13) fail to produce Th2 cells. Recently, we and others have shown that IL-4-induced phosphorylation of STAT6 and Jak1 was detected within a few minutes in Th2 cell clones and Th2 cells differentiated in vitro, whereas their phosphorylation was not detected in Th1 cells (14, 15). Thus, it is conceivable that the activation levels of signaling events downstream of IL-4R are critical for Th2 cell differentiation and for maintaining the Th2 phenotype with IL-4 production. Antigen recognition by T cell antigen receptors (TCR) is also indispensable for both Th1 and Th2 cell differentiation (3). We have reported that there is a preferential requirement for the activation of a primary tyrosine kinase, p56LCK (Lck), for TCR-mediated signal transduction in Th2 cell differentiation (16). Little is known, however, about which downstream signal-transduction pathways of Lck, such as the Ras/mitogen-activated protein kinase (MAPK) pathway, are critical for Th2 cell differentiation and how these downstream signaling molecules regulate Th2 cell differentiation.
Here, we show that TCR-mediated activation of the Ras/MAPK pathway is required for Th2 cell differentiation and that the activation of the Ras/MAPK pathway alters IL-4R function directly, probably by up-regulating the kinase activity of Jak1, which enhances STAT6 tyrosine phosphorylation. Thus, Th2 cell differentiation seems to be regulated by “crosstalk” between the TCR-mediated activation of the Ras/MAPK pathway and the IL-4R-mediated STAT6 pathway.
MATERIALS AND METHODS
Animals.
C57BL/6 (B6) and BALB/c mice were purchased from CLEA Japan (Osaka). A T cell-specific H-ras dominant-negative (dnRas) transgenic (Tg) mouse with the lck proximal promoter has been described (17). The Tg mouse line, which we used to generate substantial numbers of mature thymocytes and splenic T cells, had relatively low copy numbers of the transgene. A significant level of lck proximal promoter activity was detected in mature T cells (18). Anti-ovalbumin (OVA)-specific TCRαβ (DO10) Tg mice (19) were provided by Dennis Loh (Nippon Roche Research Center, Kanagawa, Japan). All mice used in this study were maintained under specific pathogen-free conditions.
T Cell Purification.
CD4+ T cells with naive phenotype (CD44low) were isolated from spleens on a FACSVantage cell sorter (Becton Dickinson) as described (16), yielding purity of >98%. Where indicated, naive (CD44low) CD4+ T cells from the spleen were prepared as follows. Splenocytes were incubated with culture supernatant of both anti-CD8 (53-6.72) and anti-CD44 (IM7) mAbs (PharMingen). The treated cells were washed and then incubated on plastic dishes coated with goat anti-mouse IgGs (which crossreact with rat IgG, including 53-6.72 and IM7). The nonadherent cells were used as a CD44low T cell population. Contamination of naive CD44low CD4+ cells and CD8 T cells was less than 3%.
Immunofluorescent Staining and Flow Cytometry Analysis.
In general, one million cells were incubated on ice for 30 min with the appropriate staining reagents, according to a standard method (20). For IL-4R staining, naive CD4 T cells were cultured for 2 days with IL-4 (100 units/ml), immobilized anti-TCR (H57-597; 30 μg/ml) and anti-IL-4, or immobilized anti-TCR and IL-4. Then, the cells were incubated with anti-IL-4Rα mAb (Genzyme), followed by anti-rat Ig labeled with fluorescein isothiocyanate and anti-CD4 labeled with phycoerythrin (GK1.5-PE, PharMingen). Flow cytometry analysis was performed on FACSort and FACSVantage (Becton Dickinson), and results were analyzed with cellquest software (Becton Dickinson). The free intracellular calcium ion concentration was measured as described (20).
Intracellular Staining of IL-4 and IFN-γ.
Intracellular staining of IL-4 and IFN-γ was performed as described (16). Briefly, the cultured T cells were restimulated with anti-TCR mAb (H57-597; 30 μg/ml; ref. 21) for 6 h in the presence of 2 μM monensin, which inhibited the secretion of newly produced protein. Then, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature and made permeable with 0.5% Triton X-100 (in 50 mM NaCl/5 mM EDTA/0.02% NaN3, pH 7.5) for 10 min on ice. After blocking with 3% BSA in PBS for 15 min, cells were incubated on ice for 45 min with anti-IFN-γ labeled with fluorescein isothiocyanate (XMG1.2-FITC) and anti-IL-4 labeled with phycoerythrin (11B11-PE), which were purchased from PharMingen. The stained cells were washed extensively with PBS supplemented with 1% fetal calf serum and 0.1% NaN3.
Immunoprecipitation and Immunoblotting.
In all experiments, cells were stimulated with IL-4 (100 units/ml) for 5 min at 37°C, and the reactions were terminated by adding a 10-fold volume of ice-cold PBS. Cells were harvested, washed two times with ice-cold PBS, and made soluble in lysis buffer (50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM NaF/1 mM Na3VO4/1 mM EGTA/1% Nonidet P-40/0.25% sodium deoxycholate/1 mM phenylmethylsulfonyl fluoride/1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml pepstatin). Antiserum was added to each sample, and samples were incubated at 4°C overnight. Subsequently, 50 μl of protein G-Sepharose was added, and samples were agitated for an additional 2 h at 4°C. After washing the beads three times with lysis buffer, the precipitates were resuspended in 50 μl of 2× Laemmli sample buffer containing 5% 2-mercaptoethanol, boiled for 5 min, and run on SDS/7.5% PAGE. Proteins were transferred to a poly(vinylidene difluoride) membrane and then subjected to immunoblotting with anti-phosphotyrosine (RC20; Transduction Laboratories, Lexington, KY) or with antiserum reactive with STAT6 (R&D systems), Jak1, or Jak3 (Upstate Biotechnology) or a mAb reactive with IL-4Rα (Genzyme).
MAPK Assay.
CD4 T cells were treated with anti-TCR and anti-CD4 mAb and then crosslinked with goat anti-hamster Ig antibodies. After stimulation, cells were solubilized in lysis buffer (50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM NaF/10 mM Na3VO4/2 mM EDTA/1% digitonin/40 μg/ml aprotinin/20 μg/ml leupeptin), after which 0.35 × 107 cell equivalents were reduced with 50 mM DTT and separated on an SDS/12.5% polyacrylamide gel. Proteins were transferred to a poly(vinylidene difluoride) membrane and then subjected to immunoblotting by using a phospho-MAPK detection kit (9100; New England Biolabs).
Proliferation Assay.
Naive and cultured CD4 T cells were stimulated for 40 h in 200 μl of culture medium containing 100 units/ml of recombinant IL-4. For the last 16 h, [3H]thymidine (0.5 μCi per well) was added to the stimulation culture, and the incorporated radioactivity was measured by using a β-plate (16).
Measurement of 2,4,6-Trinitrophenyl (TNP)-Specific Igs by ELISA.
The heterozygous dnRas Tg mice with (B6 × BALB/c)F1 background were immunized with 100 μg of TNP15-keyhole limpet hemocyanin in complete Freund’s adjuvant. The concentration of anti-TNP antibody (IgG1 and IgG2a) in the serum was measured by ELISA with horseradish peroxidase-conjugated anti-mouse IgG1 (Zymed) or anti-mouse IgG2a (Zymed), respectively (16). For measurements of IgE, OVA (20 μg) in alum was used to immunize mice, after which the serum concentration of OVA-specific IgE was determined with OVA-coated 96-well plates and anti-IgE mAb (Yamasa Shoyu, Chosin, Japan).
RESULTS AND DISCUSSION
Signal Transduction Through TCR and IL-4R in Naive CD4 T Cells from dnRas Tg Mice.
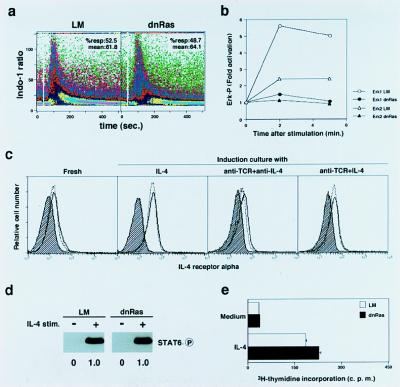
In this paper, we assessed the role of the Ras/MAPK pathways on Th2 cell differentiation by using a Tg mouse model system that specifically directed expression of a dnRas (pRHLN17) transgene in T cells (17). We used a Tg mouse line that expressed relatively low copy numbers of the transgene, and only a weak inhibition of positive selection of T cells in the thymus was observed (data not shown). In addition, slightly decreased but substantial numbers of splenic CD4 T cells were detected in the dnRas Tg mice. In the spleen of dnRas Tg mice, naive (CD44low) CD4+ T cells show a normal range of surface phenotypes, such as usage of the variable region of the TCRβ chain and expression levels of TCRαβ, CD3ɛ, CD4, CD25, and CD69 (data not shown). First, we assessed the TCR-mediated signal-transduction pathways in T cells from dnRas Tg mice. As shown in Fig. 1a, freshly isolated splenic CD4 T cells responded normally to TCR crosslinking and mobilized intracellular calcium. Anti-TCR-mAb-induced TCR-ζ phosphorylation was not impaired (data not shown). In marked contrast, the activation of MAPK kinase (MAPKK) after anti-TCR stimulation, as determined by MAPK (Erk1 and Erk2) phosphorylation, was compromised severely (Fig. 1b). Anti-TCR-induced proliferative responses and IL-2 production of naive CD4 T cells of dnRas Tg mice were decreased slightly, whereas the levels of IL-4 and IFN-γ production were not decreased but increased (data not shown).
Figure 1.
Signal transduction through TCR and IL-4R in naive CD4 T cells from dnRas Tg mice. (a) Intracellular free-calcium ion levels after TCR crosslinking (at white gap) were measured by flow cytometric analysis of Indo-1-labeled naive CD4 T cells of dnRas Tg mice and littermate (LM) controls. The mean ratio of violet to blue fluorescence of Indo-1 is plotted versus time after stimulation. Shown are data obtained by gating electronically on CD4 T cells. The percentages of responding cells and mean percentages are also shown. (b) Phosphorylation status of MAPK (Erk1 and Erk2) was examined 2 min and 5 min after TCR crosslinking by using a phospho-MAPK detection kit. Shown is the relative increase (fold) in phosphorylation of Erk1 and Erk2 with respect to unstimulated control cells (time 0). Densitometric measurement was used for the quantification; three independent experiments were done, and similar results were obtained. (c) Cell-surface expression of IL-4Rα chain on naive CD4 T cells from Tg− LM mice (dotted line) and from dnRas Tg mice (solid line) was determined after a 2-day induction culture with indicated stimulants. Background staining is shown (hatched areas). (d) Tyrosine phosphorylation of STAT6 in response to IL-4 in naive CD4 T cells from dnRas Tg mice. Freshly prepared CD44low T cells were stimulated with IL-4 (100 units/ml) for 5 min, and the tyrosine phosphorylation status of STAT6 was assessed by immunoblotting with anti-phosphotyrosine mAb (RC20). Arbitrary densitometric units are shown under each band. (e) Normal proliferative response to IL-4 (100 units/ml for 40 h) by naive CD4 T cells from dnRas Tg mice.
Next, we investigated both proximal and distal signaling events mediated by the IL-4R in naive CD4 T cells from dnRas Tg mice. Naive CD4 T cells expressed equivalent levels of IL-4Rα chain, and its expression was up-regulated normally with IL-4 stimulation (Fig. 1c). Similarly, no significant difference in IL-4Rα expression was detected after stimulation with either anti-TCR and anti-IL-4 or anti-TCR and IL-4. The amounts of STAT6 protein (data not shown) and IL-4-induced STAT6 phosphorylation were normal (Fig. 1d). IL-4-induced proliferation was also equivalent (Fig. 1e). In addition, anti-TCR-induced phosphorylation of JNK and SAPK was not impaired in dnRas Tg naive CD4 T cells (data not shown). Thus, downstream signal-transduction pathways of TCR and IL-4R in naive CD4 T cells of dnRas Tg mice seemed to be normal, except the TCR-mediated Ras/MAPK pathway.
Requirement for Activation of Ras/MAPK Pathway in Th2 Cell Differentiation in Vitro and in Vivo.
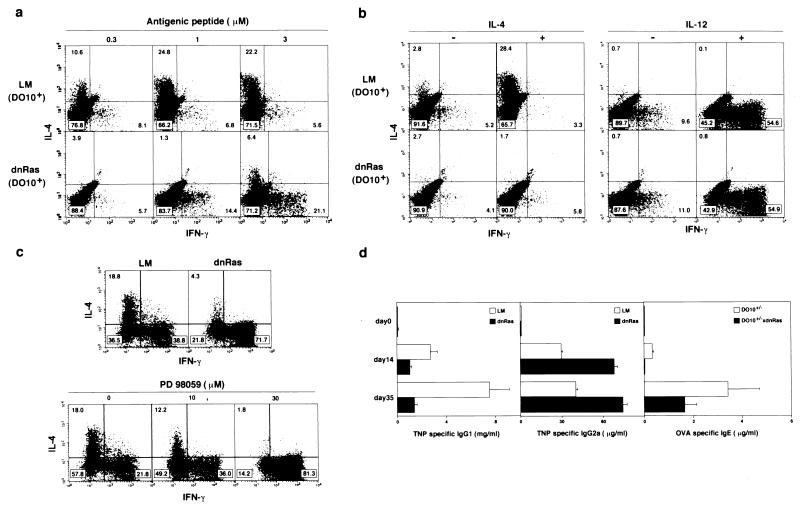
The requirement for activation of Ras/MAPK signaling pathways in Th1/Th2 cell differentiation was examined through the use of in vitro induction culture systems (16). Naive CD4 T cells from dnRas Tg mice crossed with DO10 Tg mice (19) were stimulated with antigenic OVA peptide in the presence of antigen-presenting cells for 5 days. T cells from DO10 Tg mice that are not dnRas Tg LM preferentially differentiated into IL-4-producing Th2 cells in an antigen-dose-dependent fashion (Fig. 2a Upper). However, in dnRas × DO10 double-Tg mice, development of Th2 cells was compromised severely, and a significant increase in the number of Th1 T cells was observed (Fig. 2a Lower). The impairment of Th2 cell differentiation was confirmed by measuring another Th2 cytokine, IL-5, by ELISA (data not shown). Th2 cell differentiation induced by a minimal dose of antigenic peptide and exogenous IL-4 was also inhibited in dnRas × DO10 double-Tg mice (Fig. 2b Left). Because the activation of Ras/MAPK does not seem to be involved in the IL-4R-mediated signal-transduction pathway (22) and IL-4R function is normal in dnRas Tg T cells (Fig. 1), the observed blocking effect seems to be caused by a consequence of TCR-mediated Ras/MAPK activation. In contrast, IL-12-dependent induction of Th1 cell differentiation remained intact (Fig. 2b Right). Both Th1 and Th2 cells were generated when naive CD4 T cells from non-TCR Tg (B6 × BALB/c)F1 LM mice were stimulated with anti-TCR mAb in the presence of exogenous IL-2 and IL-4 (Fig. 2c). In this condition, the generation of Th2 cells was inhibited significantly in dnRas Tg culture, whereas that of Th1 cells was enhanced significantly (31.8% vs. 71.7%). To exclude the possibility that the observed blocking effect on Th2 cell differentiation is caused by a skewed development and an accumulation of Th1 cell precursors in dnRas Tg mice, we extended the analysis by using normal (B6 × BALB/c)F1 mice and a specific inhibitor of MEK (MAPKK), PD98059 (23, 24). As we expected, the addition of 10 μM and 30 μM PD98059 to the induction culture blocked Th2 cell differentiation in a dose-dependent manner (Fig. 2c Lower). Thus, these results clearly show that the TCR-mediated activation of Ras/MAPK pathway was required for Th2 differentiation in vitro. The enhanced generation of Th1 cells observed in dnRas Tg T cell cultures or normal T cell cultures treated with PD98059 could be a consequence of a “default” pathway of Th1 cell differentiation as proposed (25). Wortmannin (a phosphatidylinositol 3-kinase inhibitor) and SB203580 (a p38 MAPK inhibitor) had no effect on Th2 cell differentiation (data not shown). However, it is still possible that other Ras-induced signaling pathways may involved in the differentiation process of Th2 cells in addition to the Erk MAPK pathway.
Figure 2.
Requirement for activation of Ras/MAPK pathway in Th2 cell differentiation in vitro and in vivo. (a) Naive CD4 T cells from dnRas × DO10 double-Tg mice were stimulated with antigenic peptide (OVA; 323-339) and irradiated BALB/c (H-2d) antigen-presenting cells for 5 days, and intracellular production of IFN-γ and IL-4 was detected. (b) Naive T cells were stimulated with either a minimal dose (0.3 μM) of antigenic peptide and exogenous IL-4 (100 units/ml; Left) or with 1 μM antigenic peptide in the presence of anti-IL-4 mAb and exogenous IL-12 (0.1 unit/ml; Right). (c) Th1/Th2 cell differentiation of naive T cells from dnRas Tg mice with (B6 × BALB/c)F1 background and the effect of a specific inhibitor of MEK (MAPKK), PD 98059. Naive CD4 T cells from normal and dnRas Tg mice were stimulated with immobilized anti-TCR in the presence of IL-2 (30 units/ml) and IL-4 (100 units/ml) for 2 days and then cultured in the medium with the same concentrations of IL-2 and IL-4 for another 3 days. The cultured cells were subjected to intracellular staining with anti-IL-4 and anti-IFN-γ. (d) Effect of overexpression of dnRas on helper T cell differentiation in vivo. Tg− LM mice and dnRas Tg mice were primed on day 0 and boosted on day 21; 2 weeks after each immunization, serum concentrations of antigen-specific antibodies were determined by ELISA. Mice with B6 backgrounds were immunized with TNP-keyhole limpet hemocyanin (100 μg per mouse) in complete Freund’s adjuvant, and serum concentrations of TNP-specific IgG1 and IgG2a were measured (Left and Center). DO10 × dnRas Tg− control (D10+/−) and DO10 × dnRas double-Tg (D10+/− × dnRas) mice were immunized with OVA (100 μg per mouse) in alum, and serum concentrations of OVA-specific IgE were measured (Right). Bars depict mean values of four animals with standard deviations expressed as error bars.
Th2 cells play an important role in stimulating B cells to produce high levels of antigen-specific IgG1 and IgE in vivo, whereas the IgG2a isotype seems to be a consequence of Th1 cell differentiation (26). Consequently, we wished to assess the role for Ras activation in Th2 cell differentiation in vivo by using dnRas Tg mice and assessing the isotype of antigen-specific antibodies. Control LM mice and dnRas Tg mice were immunized with TNP-keyhole limpet hemocyanin, and serum concentrations of TNP-specific IgG1 and IgG2a were measured. The serum concentrations of Th2 cell-dependent isotype IgG1 were significantly lower in dnRas Tg mice (Fig. 2d Left). Th1-dependent IgG2a levels were not decreased but increased about 2-fold (Fig. 2d Center). In addition, the serum concentration of OVA-specific IgE was decreased significantly in dnRas Tg mice (Fig. 2d Right). These results are consistent with substantial impairment of antigen-specific Th2 cell differentiation in vivo in dnRas Tg mice, whereas Th1 cell differentiation remained intact and was enhanced.
Improvement of IL-4R Function After TCR Stimulation for 2 Days.
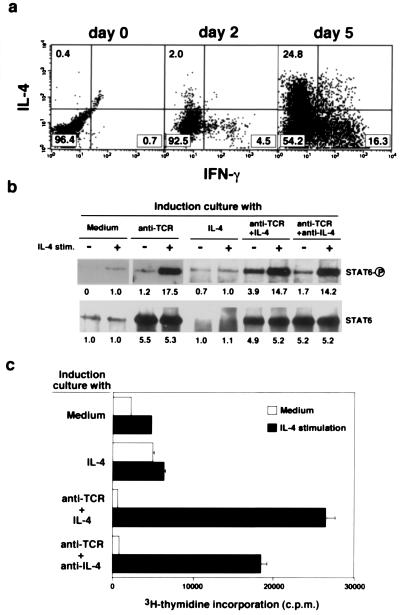
For Th2 cell differentiation, TCR-mediated activation of the Ras/MAPK pathway and IL-4-mediated activation of STAT6 are required. The IL-4 treatment of naive T cells without TCR ligation does not induce Th2 cell differentiation (3), and IL-4 receptor function differs between established Th1 and Th2 clones (14, 15). Therefore, TCR-mediated activation of the Ras/MAPK pathway may alter IL-4R-mediated signaling. Consequently, we investigated whether TCR stimulation induced changes in IL-4R-mediated signaling. Fig. 3a shows the generation of Th1 and Th2 cells from naive T cells 2 days or 5 days after TCR stimulation in the presence of IL-2 and IL-4. At 2 days, only a few T cells express Th1 or Th2 phenotype. However, IL-4-induced phosphorylation of STAT6 protein was enhanced dramatically (1.0% vs. 17.5%) in the cells stimulated with anti-TCR for 2 days (induction culture; Fig. 3b Left). The expression levels of STAT6 protein also were increased with TCR stimulation in the induction culture. Interestingly, the enhancement was not affected by the depletion of IL-4 from the induction culture caused by the neutralizing antibody (Fig. 3b Right), indicating that the changes were caused by TCR-mediated consequences. Consistent with the phosphorylation levels of STAT6, IL-4-induced proliferation was improved greatly when naive T cells received TCR-mediated stimulation (Fig. 3c). Although the treatment with IL-4 alone increased cell-surface expression levels of IL-4R (Fig. 1c), IL-4R function was not improved in terms of IL-4-dependent proliferation (Fig. 3c).
Figure 3.
Improvement of IL-4R-mediated signal transduction after TCR stimulation. (a) Anti-TCR-induced Th1/Th2 cell differentiation on day 2 and day 5. Anti-TCR-stimulated naive CD4 T cells from (B6 × BALB/c)F1 mice were harvested on day 0, day 2, and day 5. The percentages of cells in each area are shown. (b) Naive CD4 T cells from (B6 × BALB/c)F1 mice were cultured for 2 days with medium, immobilized anti-TCR mAb, IL-4 alone, immobilized anti-TCR mAb and IL-4, or immobilized anti-TCR and anti-IL-4 mAb (induction culture). The percentages of recovered live cells in these cultures were 92%, 123%, 89%, 120%, and 135%. The induced cells were cultured without IL-4 or anti-IL-4 for another 8 h at 37°C, and then the cells were stimulated with IL-4 (100 units/ml) for 5 min. The phosphorylation status of STAT6 and the amount of STAT6 protein were assessed by immunoprecipitation with anti-STAT6 mAb and, after immunoblotting, with anti-phosphotyrosine or anti-STAT6 mAb. The equivalent of 20 million cells was loaded in each lane. (c) In vitro cultured cells like those in b were stimulated with IL-4 (100 units/ml) for 40 h, and [3H]thymidine incorporation (over the last 16 h) was measured.
Involvement of Ras/MAPK Pathway in the Improvement of IL-4R Function.
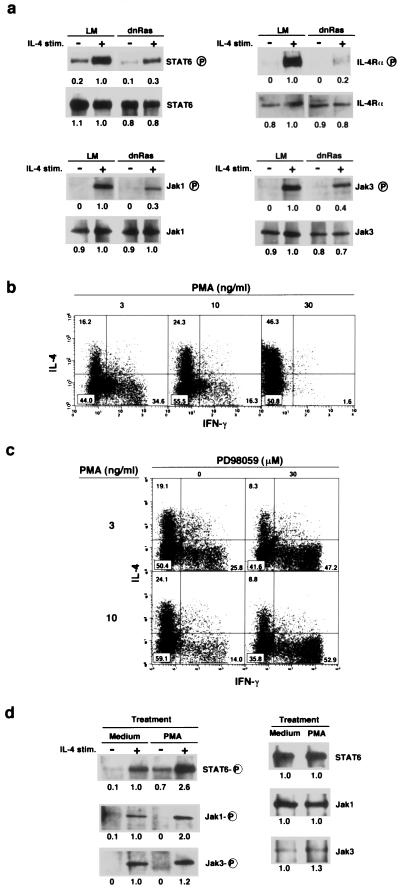
Consequently, we wished to assess the involvement of TCR-mediated Ras/MAPK activation in the improvement of IL-4R function. Naive CD4 T cells were prepared from dnRas Tg mice with panning and cultured with anti-TCR mAb in the presence of IL-2 and IL-4 for 2 days, and then the phosphorylation status of STAT6, IL-4Rα, Jak1, and Jak3 was assessed after 5 min of IL-4 stimulation. As shown in Fig. 4a, all of these signaling molecules were tyrosine phosphorylated after 5 min of IL-4 stimulation in Tg− LM mice. However, their phosphorylation was decreased substantially in dnRas Tg mice. The decrease was most dramatic in IL-4Rα and Jak1 with respect to the amount of protein present in the precipitates. During the first 2 days of the induction culture with anti-TCR, protein expression levels of STAT6 (Fig. 3b), IL-4Rα, Jak1, and Jak3 (data not shown) were increased. Therefore, TCR-mediated signals induced two different effects on IL-4R-mediated signal transduction: an increase in the amount of STAT6, Jak1, and Jak3 protein and increased susceptibility in the phosphorylation of these molecules. The latter seemed to be a consequence of the activation of the Ras/MAPK signaling pathway.
Figure 4.
IL-4-induced phosphorylation on STAT6 and Jak1 in naive T cells was regulated by the Ras/MAPK signaling pathway. (a) Naive CD4 T cells from dnRas Tg mice were cultured for 2 days with immobilized anti-TCR mAb and IL-4 and cultured without IL-4 for 8 h. Then, IL-4-induced phosphorylation on STAT6, IL-4Rα, Jak1, and Jak3 was assessed by immunoprecipitation with specific mAb for each protein and, after immunoblotting, with anti-phosphotyrosine mAb. Arbitrary densitometric units are shown under each band. The amount of protein was determined also by reblotting the same membrane with specific mAbs. (b) Phorbol 12-myristate 13-acetate (PMA) dose-dependent increase in the generation of Th2 cells in vitro. Naive T cells from (B6 × BALB/c)F1 mice were treated with indicated doses of PMA for 2 days in the presence of ionomycin (300 nM), IL-2 (30 units/ml), and IL-4 (100 units/ml). (c) Effect of PD98059 on the PMA-induced Th1/Th2 cell differentiation. (d) Naive CD4 T cells were treated with PMA (30 ng/ml) for 4 h, and IL-4-induced phosphorylation on STAT6, Jak1, and Jak3 was assessed by the same method used in a. The amount of each protein existing in the cells with or without PMA treatment was also determined (Right).
To investigate further the molecular mechanism regulating IL-4R-mediated signal transduction by the Ras/MAPK pathway, we used PMA as an activating agent on the Ras/MAPK pathway. First, the effect of PMA on Th1/Th2 cell differentiation was assessed. Naive CD4 T cells from normal (B6 × BALB/c)F1 mice were stimulated with various concentrations of PMA in the presence of 300 nM ionomycin (Fig. 4b). Consistent with the results obtained thus far, a PMA dose-dependent increase in the generation of Th2 cells and a decrease in Th1 cell differentiation were observed. Fig. 4c shows that the PMA-induced Th2 cell differentiation was blocked substantially by PD98059. Again, the enhancement of Th1 cell differentiation was observed. These results suggest that the observed effect of PMA was a MEK (MAPKK)-dependent phenomenon. Next, naive CD4 T cells were treated with PMA for 4 h, and IL-4-mediated phosphorylation of STAT6, Jak1, and Jak3 was assessed (Fig. 4d). The levels of phosphorylation of STAT6 and Jak1 clearly were increased when naive T cells were treated with PMA. The increase was detected even 15 min after the treatment (data not shown) and was reproducible in three independent experiments. Because protein expression levels of STAT6, Jak1, and Jak3 were not changed by the 4-h treatment with PMA (Fig. 4d Right), the enhancement of tyrosine phosphorylation of these molecules was not a result of up-regulation of transcription or translation. Thus, Jak1 and/or STAT6 seemed to be targets of posttranslational regulation by the Ras/MAPK signaling pathway.
In this report, we show that the TCR-mediated activation of Ras/MAPK pathway is required for Th2 cell differentiation and that Th1 and Th2 cell differentiation exhibit differential dependence on activation of the Ras/MAPK pathway. Th2 cell differentiation was impaired in the dnRas Tg T cells; however, Th1 cell differentiation remained intact, and sometimes it was even enhanced (Fig. 2). Several recent reports have suggested that low-dose infection or low antigen concentration favors induction of Th1 responses, whereas high-dose antigenic stimulation preferentially induces Th2 responses (27–29). Taken together with our findings, these results suggest that weak antigenic stimulation may not activate the Ras/MAPK pathway efficiently and thus may favor Th1 cell differentiation, whereas strong stimulation with higher concentrations of antigenic peptide may be required to activate the Ras/MAPK pathway sufficiently to induce Th2 cell differentiation. This explanation is consistent with the results obtained from our previous analysis of Th1/Th2 differentiation in the dominant-negative Lck Tg mice (16). Thus, the extent to which the Ras/MAPK pathway is activated seems to determine the lineage (Th1 or Th2 cells) into which naive T helper cells will differentiate. Because various allergic diseases are closely related to Th2 dominant immune responses (2, 3), it is conceivable that the down-regulation of activation in the Ras/MAPK pathway could result in decreased susceptibility to allergic diseases. Recently, the requirement for the JNK MAPK pathway in Th1 cell differentiation has been reported (30). Thus, the determination of Th1 or Th2 cell differentiation in naive CD4 T cells in the periphery seems to depend on the activation of distinct MAPK signaling pathways.
Second, IL-4R function on naive T cells is found to be up-regulated by TCR-mediated activation of the Ras/MAPK pathway. From our results with short-term PMA treatment (Fig. 4d), the qualitative improvement in IL-4R-mediated signal transduction is caused in part by posttranslational regulation of Jak1 and/or STAT6 molecules by the Ras/MAPK signaling pathway. Although the precise molecular mechanism by which the Ras/MAPK pathway regulates the Jak1/STAT6 pathway remains unclear, one possibility is that activated MEK (MAPKK) phosphorylates and up-regulates Jak1 activity. In fact, there is a potential tyrosine phosphorylation site of MEK (MAPKK), “TEY” (31), and a threonine residue as part of the XPXS/TP motif (32) in mouse Jak1. Our preliminary experiments with anti-phosphotyrosine mAb specific for TEY suggested that short-term PMA stimulation caused phosphorylation of TEY on Jak1 molecules (data not shown). Recently, Petricoin et al. (33) reported that the antiproliferative action of IFN-α required components of the TCR signaling cascade, such as Lck and ZAP-70. Here, we present evidence that the TCR-mediated Ras/MAPK activation controls IL-4R-mediated signaling and determines the direction of helper T cell differentiation. Indeed, our data suggest that the lineage choices made by naive Th cells after antigen stimulation are influenced clearly by crosstalk between the antigen receptor- and cytokine receptor-mediated signaling cascades.
Acknowledgments
We thank Drs. David Wiest and Ralph T. Kubo for helpful comments during the preparation of the manuscript. We are also grateful to Dr. Masaru Taniguchi for warm-hearted support. This work was supported by grants from the Ministry of Education, Science, and Culture (Japan), by the Ministry of Health and Welfare (Japan), by the Uehara Memorial Foundation, and by the Kanagawa Academy of Science and Technology.
ABBREVIATIONS
- B6
C57BL/6
- dnRas
H-Ras dominant negative
- DO10
anti-OVA-specific TCRαβ
- IFN
interferon
- IL
interleukin
- IL-4R
interleukin 4 receptor
- Lck
lymphoid cell protein tyrosine kinase p56LCK
- LM
littermate
- MAPK
mitogen-activated protein kinase
- MAPKK
MAPK kinase
- OVA
ovalbumin
- PMA
phorbol 12-myristate 13-acetate
- TCR
T cell antigen receptor
- Tg
transgenic
- Th1
type-1 helper T
- Th2
type-2 helper T
- TNP
2,4,6-trinitrophenyl
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Seder R A, Paul W E. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 3.Abbas A K, Murphy K M, Sher A. Nature (London) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 4.O’Garra A. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 5.Seder R A, Gazzinelli R, Sher A, Paul W E. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guler M L, Gorham J D, Hsieh C-S, Mackey A J, Steen R G, Dietrich W F, Murphy K M. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh C-S, Heimberger A B, Gold J S, O’Garra A, Murphy K M. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Gros G, Ben-Sasson S Z, Seder R, Finkelman F D, Paul W E. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn R, Rajewsky K, Muller W. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 10.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Nature (London) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Nature (London) 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 12.Shimoda K, van Deursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A A, et al. Nature (London) 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan M H, Schindler U, Smiley S T, Grusby M J. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 14.Kubo M, Ransom J, Webb D, Hashimoto Y, Tada T, Nakayama T. EMBO J. 1997;16:4007–4020. doi: 10.1093/emboj/16.13.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Paul W E. J Exp Med. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita M, Hashimoto K, Kimura M, Kubo M, Tada T, Nakayama T. Int Immunol. 1998;10:577–591. doi: 10.1093/intimm/10.5.577. [DOI] [PubMed] [Google Scholar]
- 17.Swan K A, Alberola-Ila J, Gross J A, Appleby M W, Forbush K A, Thomas J F, Perlmutter R M. EMBO J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen J M, Forbush K A, Perlmutter R M. Mol Cell Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama T, June C H, Munitz T I, Sheard M, McCarthy S A, Sharrow S O, Samelson L E, Singer A. Science. 1990;249:1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- 21.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 22.Welham M J, Duronio V, Schrader J W. J Biol Chem. 1994;269:5865–5873. [PubMed] [Google Scholar]
- 23.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh C-S, Macatonia S E, O’Garra A, Murphy K M. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffman R L, Lebman D A, Rothman P. Adv Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 27.Hosken N A, Shibuya K, Heath A W, Murphy K M, O’Garra A. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burstein H J, Abbas A K. J Exp Med. 1993;177:457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Proc Natl Acad Sci USA. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D D, Conze D, Whitmarsh A J, Barrett T, Davis R J, Rincon M, Flavell R A. Immunity. 1988;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 31.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 32.Wen Z, Zhong Z, Darnell J E., Jr Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 33.Petricoin E F, III, Ito S, Williams B L, Audet S, Stancato L F, Gamero A, Clouse K, Grimley P, Weiss A, Beeler J, et al. Nature (London) 1997;390:629–632. doi: 10.1038/37648. [DOI] [PubMed] [Google Scholar]