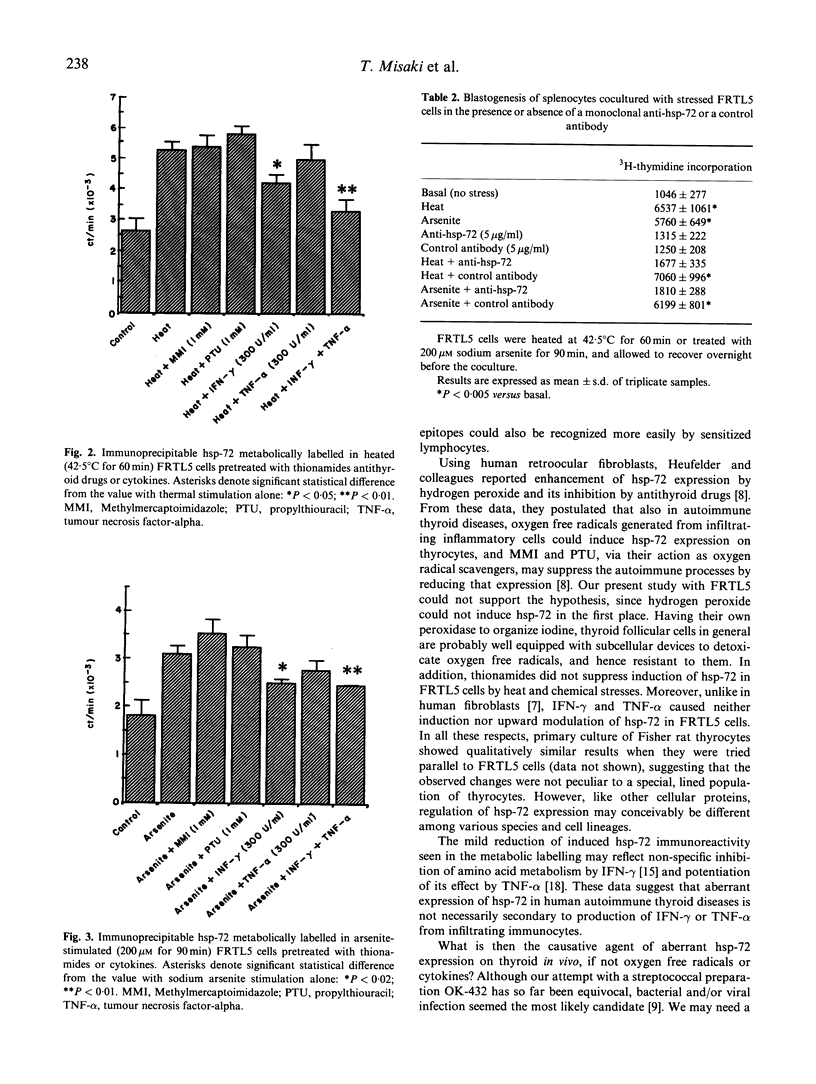
Abstract
In Graves' disease and Hashimoto's thyroiditis, the presence of 72-kD heat shock protein (hsp-72) on thyrocytes has been reported. To clarify the significance of this phenomenon, we induced the antigen in thyroid cell culture in vitro. In the FRTL5 rat cell line, which had been heated at 42.5 degrees C or treated with sodium arsenite, expression of hsp-72 was examined with immunoperoxidase staining and immunoprecipitation of the metabolically labelled protein using a specific MoAb. In the cells cultured either with or without thyrotropin (TSH), heat and chemical stresses reproducibly and dose-dependently induced hsp-72 antigen, whereas unstimulated controls had no significant immunoreactivity. Unlike in Graves' retrocular fibroblasts, hydrogen peroxide was not an effective stress in FRTL5, and the induction was not suppressed by methylmercaptoimmidazole and propylthiouracil, nor enhanced by interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha). These data could not support the hypotheses that suppression of thyroid autoimmunity by thionamides is due to their modulatory action on hsp-72 expression, or that presence of that antigen in the thyroid tissues affected by autoimmunity is secondary to cytokine secretion from infiltrating immunocytes. On the other hand, coculture experiments of stressed FRTL5 cells and syngeneic Fisher rat splenocytes suggest that aberrantly expressed hsp may activate part of the thyroid-infiltrating lymphocytes and thereby aggravate autoimmune processes. The induction and detection systems of hsp-72 using FRTL5 cells would facilitate future studies, possibly utilizing human materials as well, to explore possible relations between stress proteins and thyroid autoimmunity.
Full text
PDF

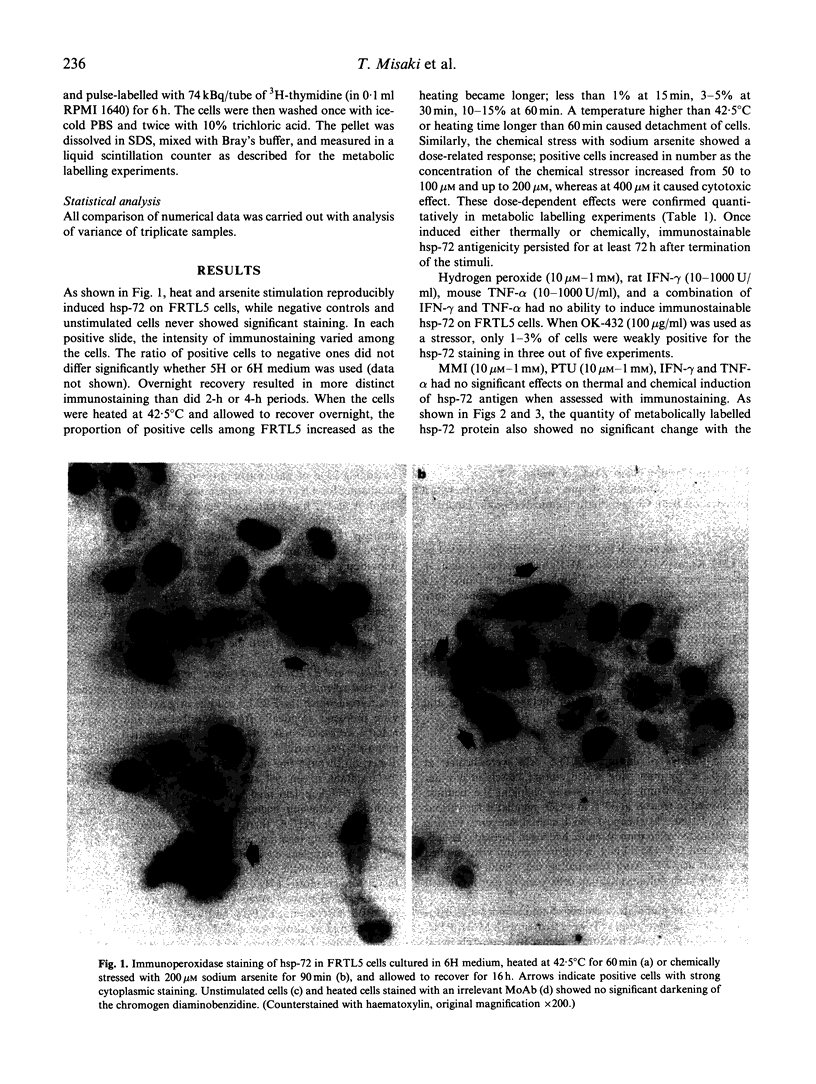
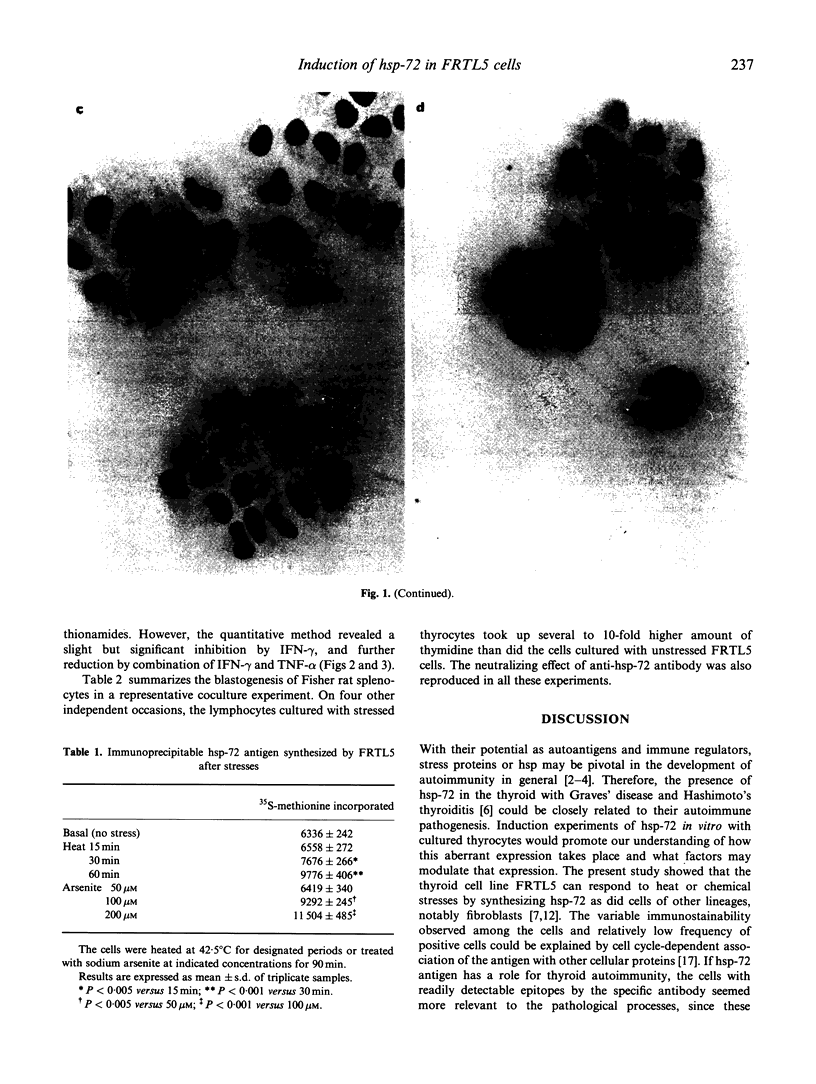

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen L., Van der Heyden J., Ruysschaert R., Fiers W. Recombinant tumor necrosis factor: its effect and its synergism with interferon-gamma on a variety of normal and transformed human cell lines. Eur J Cancer Clin Oncol. 1986 Apr;22(4):419–426. doi: 10.1016/0277-5379(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Buchan G., Chantry D., Kassal H., Londei M., Pirich K., Barrett K., Turner M., Waldhausl W., Feldmann M. Analysis of intrathyroidal cytokine production in thyroid autoimmune disease: thyroid follicular cells produce interleukin-1 alpha and interleukin-6. Clin Exp Immunol. 1989 Sep;77(3):324–330. [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Quayle A. J. Heat shock proteins: friend and foe? Clin Exp Immunol. 1991 Oct;86(1):2–5. doi: 10.1111/j.1365-2249.1991.tb05764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufelder A. E., Goellner J. R., Wenzel B. E., Bahn R. S. Immunohistochemical detection and localization of a 72-kilodalton heat shock protein in autoimmune thyroid disease. J Clin Endocrinol Metab. 1992 Apr;74(4):724–731. doi: 10.1210/jcem.74.4.1548334. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Wenzel B. E., Bahn R. S. Methimazole and propylthiouracil inhibit the oxygen free radical-induced expression of a 72 kilodalton heat shock protein in Graves' retroocular fibroblasts. J Clin Endocrinol Metab. 1992 Apr;74(4):737–742. doi: 10.1210/jcem.74.4.1532179. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Wenzel B. E., Gorman C. A., Bahn R. S. Detection, cellular localization, and modulation of heat shock proteins in cultured fibroblasts from patients with extrathyroidal manifestations of Graves' disease. J Clin Endocrinol Metab. 1991 Oct;73(4):739–745. doi: 10.1210/jcem-73-4-739. [DOI] [PubMed] [Google Scholar]
- Iida Y., Kasagi K., Tokuda Y., Arai K., Misaki T., Konishi J. Antibody-dependent cell-mediated growth inhibition of rat thyroid cells, FRTL-5, in patients with autoimmune thyroid diseases. Acta Endocrinol (Copenh) 1988 Aug;118(4):580–586. doi: 10.1530/acta.0.1180580. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- McGregor A. M. Heat shock proteins and autoimmune thyroid disease; too hot to handle? J Clin Endocrinol Metab. 1992 Apr;74(4):720–723. doi: 10.1210/jcem.74.4.1548333. [DOI] [PubMed] [Google Scholar]
- Michalek M. T., Grant E. P., Gramm C., Goldberg A. L., Rock K. L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993 Jun 10;363(6429):552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Welch W. J., Morimoto R. I. Cell cycle-dependent association of HSP70 with specific cellular proteins. J Cell Biol. 1989 Feb;108(2):413–423. doi: 10.1083/jcb.108.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki T., Konishi J., Nakashima T., Iida Y., Kasagi K., Endo K., Uchiyama T., Kuma K., Torizuka K. Immunohistological phenotyping of thyroid infiltrating lymphocytes in Graves' disease and Hashimoto's thyroiditis. Clin Exp Immunol. 1985 Apr;60(1):104–110. [PMC free article] [PubMed] [Google Scholar]
- Misaki T., Tramontano D., Ingbar S. H. Effects of rat gamma- and non-gamma-interferons on the expression of Ia antigen, growth, and differentiated functions of FRTL5 cells. Endocrinology. 1988 Dec;123(6):2849–2857. doi: 10.1210/endo-123-6-2849. [DOI] [PubMed] [Google Scholar]
- Sierra-Rivera E., Voorhees G. J., Freeman M. L. Gamma irradiation increases hsp-70 in Chinese hamster ovary cells. Radiat Res. 1993 Jul;135(1):40–45. doi: 10.2307/3578394. [DOI] [PubMed] [Google Scholar]
- Tomer Y., Davies T. F. Infection, thyroid disease, and autoimmunity. Endocr Rev. 1993 Feb;14(1):107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]