Abstract
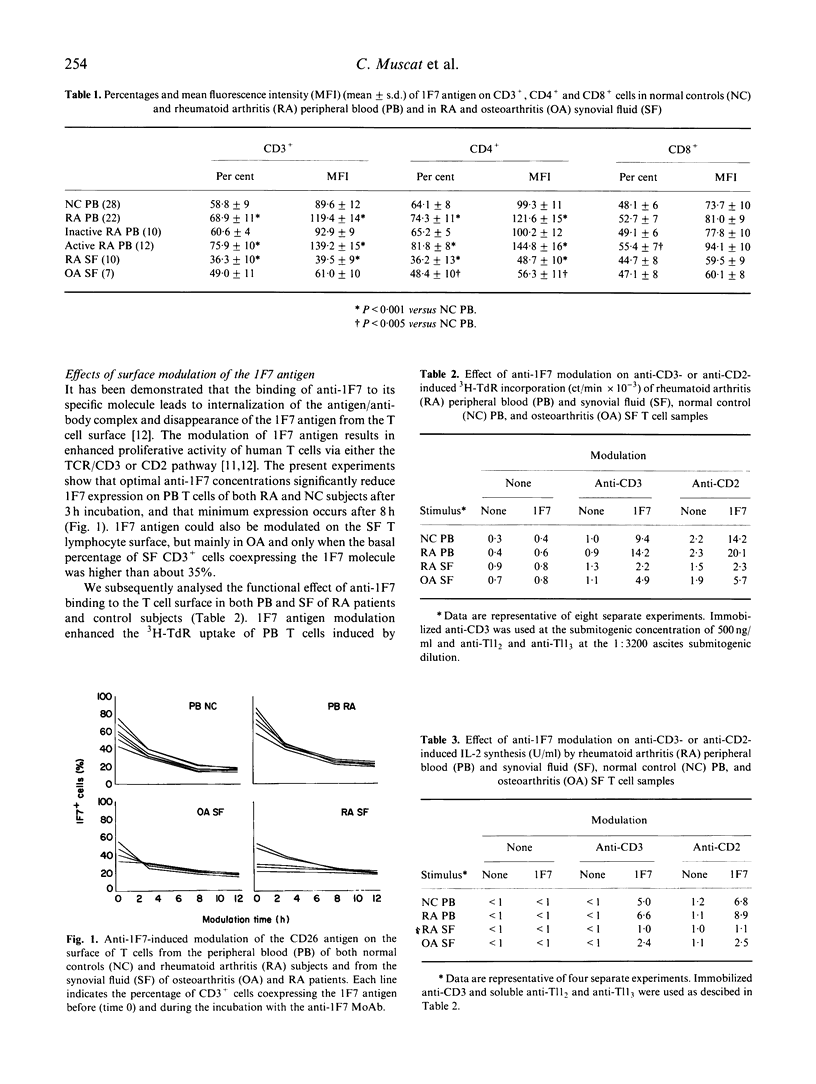
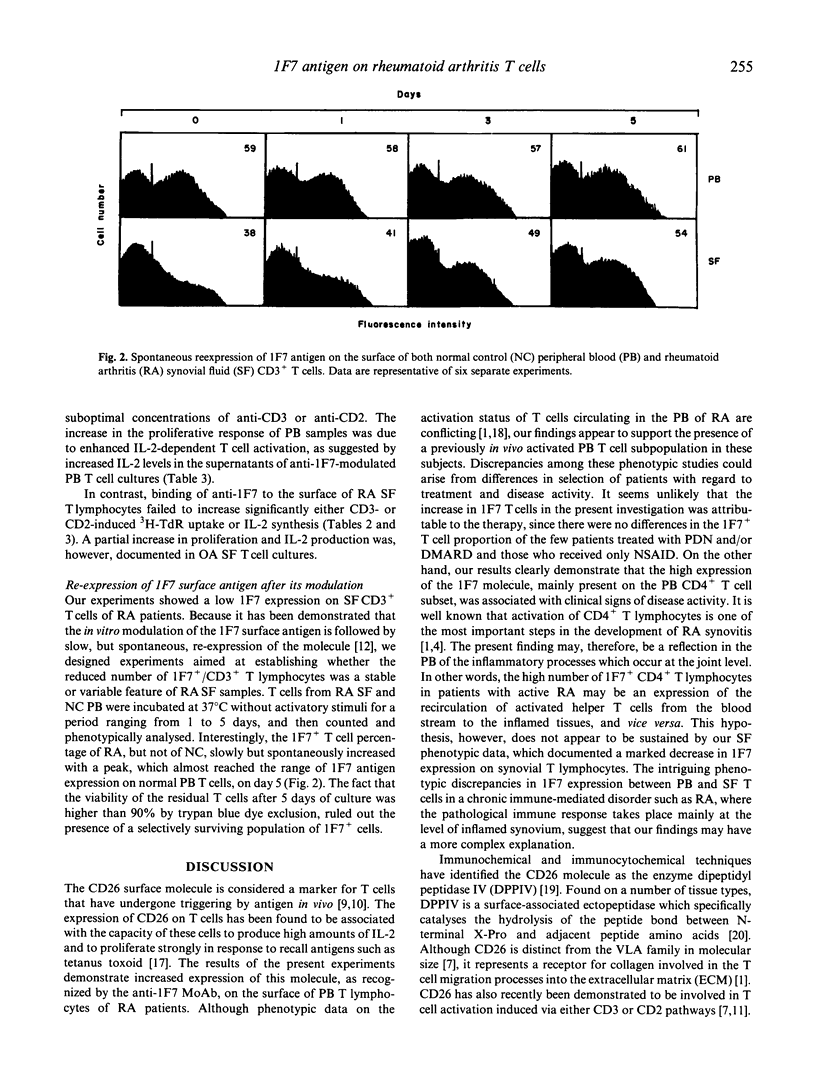
The expression and the functional role of the CD26 (1F7) T cell surface molecule, an ectoenzyme which seems to represent a functional collagen receptor of T lymphocytes and to have a role in T cell activation, were analysed in both peripheral blood (PB) and synovial fluid (SF) T cell samples from patients with active and inactive rheumatoid arthritis (RA). Although patients with active disease displayed higher percentages of PB CD26+ CD4+ T cells than inactive RA and control subjects, CD26 antigen expression on RA SF T lymphocytes was low. The anti-1F7 binding to the T cell surface, that led to CD26 antigen modulation and enhancement of both IL-2 synthesis by, and 3H-TdR incorporation of, anti-CD3- or anti-CD2-triggered PB T cells in RA and control subjects, was unable to affect significantly both expression and functional activity of RA SF T lymphocytes. Since the 1F7 antigen spontaneously reappeared on the surface of unstimulated SF T cells after 2-5 days of culturing, the low 1F7 antigen expression of anti-1F7 in the SF T cell compartment may be the result of in vivo molecule modulation exerted by the natural ligand in the joint, with important implications for T cell activation and lymphokine synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Pietschmann P., Oppenheimer-Marks N., Lipsky P. E. The intrinsic migratory capacity of memory T cells contributes to their accumulation in rheumatoid synovium. Arthritis Rheum. 1992 Dec;35(12):1434–1444. doi: 10.1002/art.1780351206. [DOI] [PubMed] [Google Scholar]
- Dang N. H., Torimoto Y., Deusch K., Schlossman S. F., Morimoto C. Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol. 1990 Jun 1;144(11):4092–4100. [PubMed] [Google Scholar]
- Dang N. H., Torimoto Y., Schlossman S. F., Morimoto C. Human CD4 helper T cell activation: functional involvement of two distinct collagen receptors, 1F7 and VLA integrin family. J Exp Med. 1990 Aug 1;172(2):649–652. doi: 10.1084/jem.172.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang N. H., Torimoto Y., Sugita K., Daley J. F., Schow P., Prado C., Schlossman S. F., Morimoto C. Cell surface modulation of CD26 by anti-1F7 monoclonal antibody. Analysis of surface expression and human T cell activation. J Immunol. 1990 Dec 15;145(12):3963–3971. [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Agea E., Lanfrancone L., Cernetti C., Spinozzi F., Rambotti P. Basis for defective proliferation of peripheral blood T cells to anti-CD2 antibodies in primary Sjögren's syndrome. J Clin Invest. 1990 Dec;86(6):1870–1877. doi: 10.1172/JCI114918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli R., Bertotto A., Rambotti P., Barbieri P., Ciompi M. L., Bombardieri S. T cell immunoregulation in rheumatoid synovitis. Arthritis Rheum. 1988 Aug;31(8):1075–1076. doi: 10.1002/art.1780310823. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Chofflon M., Benjamin D., Dang N. H., Breitmeyer J. Mechanisms of immune memory. T cell activation and CD3 phosphorylation correlates with Ta1 (CDw26) expression. J Immunol. 1989 Apr 15;142(8):2590–2596. [PubMed] [Google Scholar]
- Hanski C., Huhle T., Gossrau R., Reutter W. Direct evidence for the binding of rat liver DPP IV to collagen in vitro. Exp Cell Res. 1988 Sep;178(1):64–72. doi: 10.1016/0014-4827(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Masuyama J., Berman J. S., Cruikshank W. W., Morimoto C., Center D. M. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992 Mar 1;148(5):1367–1374. [PubMed] [Google Scholar]
- Mattern T., Scholz W., Feller A. C., Flad H. D., Ulmer A. J. Expression of CD26 (dipeptidyl peptidase IV) on resting and activated human T-lymphocytes. Scand J Immunol. 1991 Jun;33(6):737–748. doi: 10.1111/j.1365-3083.1991.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Torimoto Y., Levinson G., Rudd C. E., Schrieber M., Dang N. H., Letvin N. L., Schlossman S. F. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989 Dec 1;143(11):3430–3439. [PubMed] [Google Scholar]
- Nagatsu T., Hino M., Fuyamada H., Hayakawa T., Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem. 1976 Aug;74(2):466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Potocnik A. J., Kinne R., Menninger H., Zacher J., Emmrich F., Kroczek R. A. Expression of activation antigens on T cells in rheumatoid arthritis patients. Scand J Immunol. 1990 Feb;31(2):213–224. doi: 10.1111/j.1365-3083.1990.tb02762.x. [DOI] [PubMed] [Google Scholar]
- Ulmer A. J., Mattern T., Feller A. C., Heymann E., Flad H. D. CD26 antigen is a surface dipeptidyl peptidase IV (DPPIV) as characterized by monoclonal antibodies clone TII-19-4-7 and 4EL1C7. Scand J Immunol. 1990 Apr;31(4):429–435. doi: 10.1111/j.1365-3083.1990.tb02789.x. [DOI] [PubMed] [Google Scholar]
- Ziff M. Role of the endothelium in chronic inflammatory synovitis. Arthritis Rheum. 1991 Nov;34(11):1345–1352. doi: 10.1002/art.1780341102. [DOI] [PubMed] [Google Scholar]


