Abstract
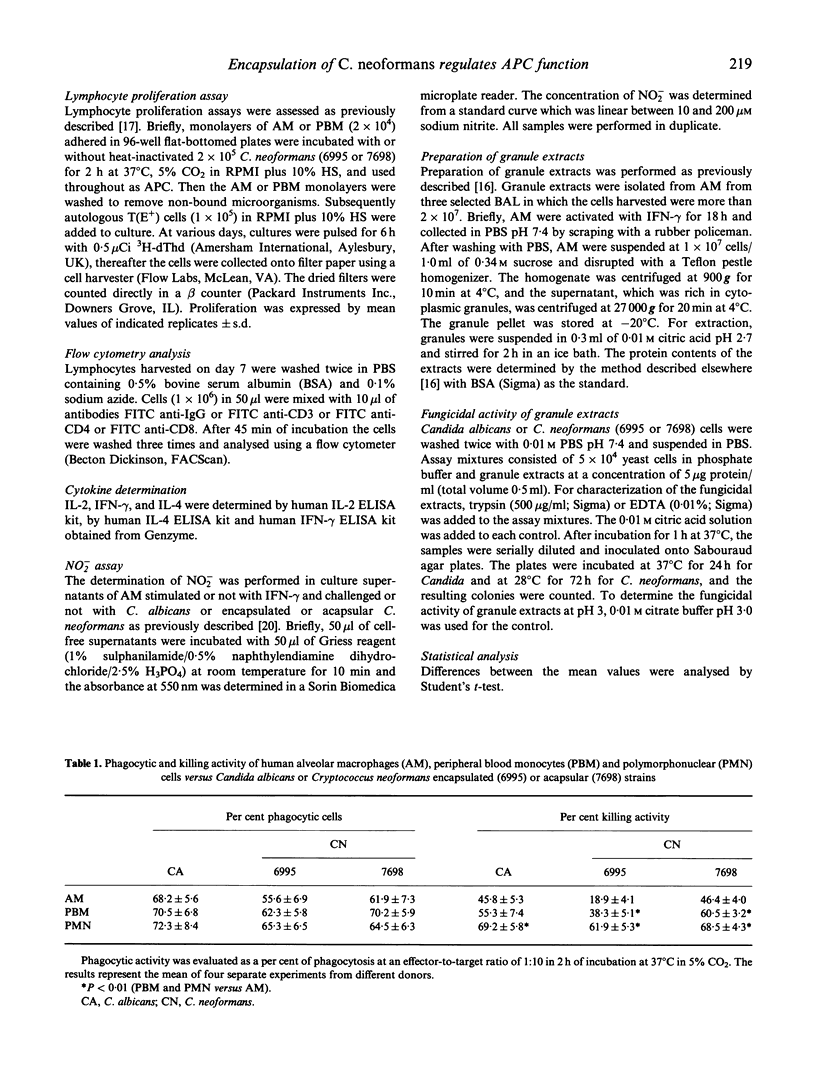
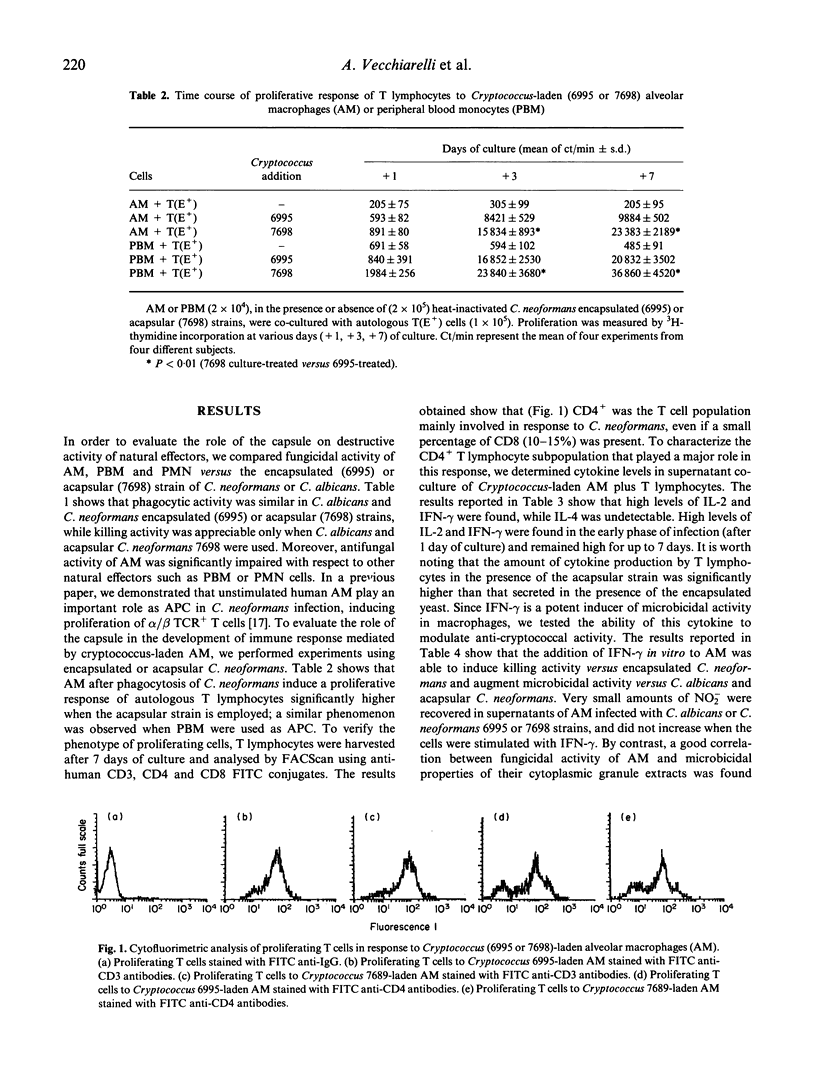
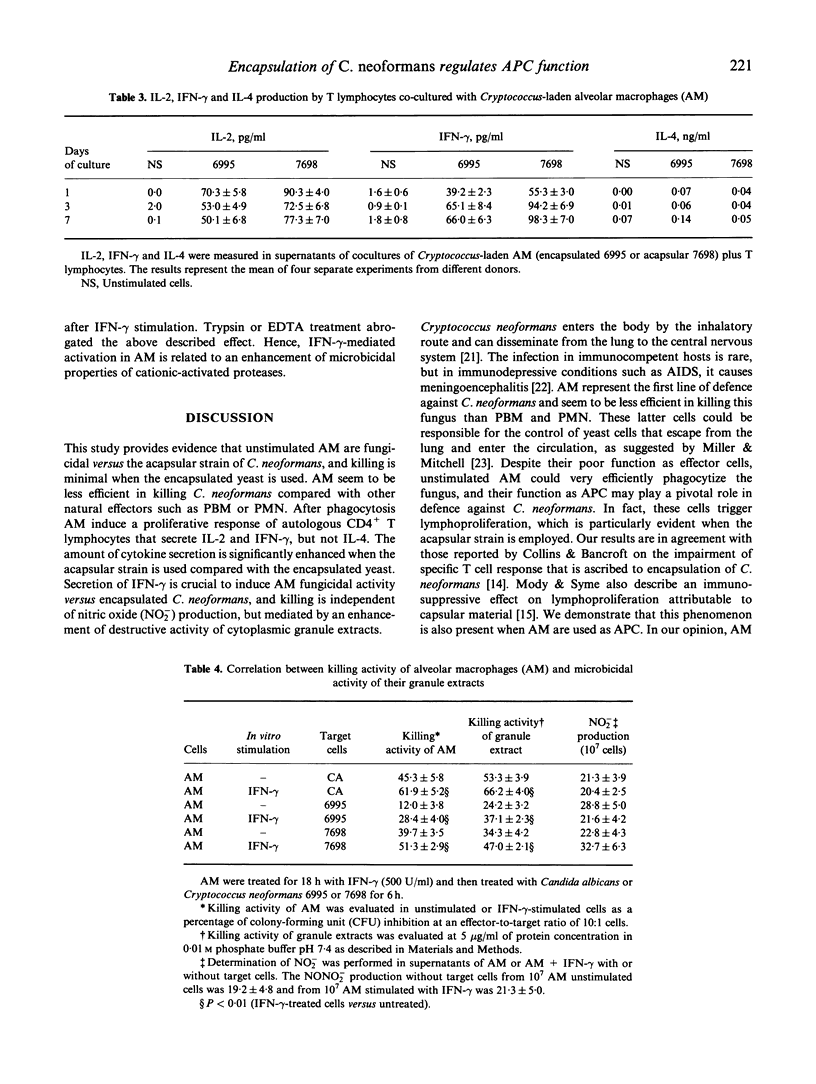
Our previous studies have shown that unstimulated alveolar macrophages (AM) play a predominant role as antigen-presenting cells in Cryptococcus neoformans infections, while the function as effector cells seems to be of minor relevance. The present study focuses on the role of encapsulation of C. neoformans on fungicidal activity and the antigen presentation process of AM. Fungicidal activity in unstimulated AM occurs to a higher degree when the acapsular strain is employed, but this is impaired compared with other natural effectors, such as peripheral blood monocytes (PBM) and polymorphonuclear (PMN) cells. Cryptococcus-laden AM also induce a higher proliferative response in autologous CD4+ lymphocytes when the acapsular strain is used compared with encapsulated yeast. The enhanced blastogenic response is, in part, ascribed to an augmented IL-2 production by T cells. In addition, higher levels of interferon-gamma (IFN-gamma), but not IL-4, are produced by the responding T cells, when the acapsular strain is used compared with the encapsulated yeast. Moreover, IFN-gamma is able to induce fungicidal activity in AM against the encapsulated yeast and augments killing activity of the acapsular strain. This phenomenon is not mediated by nitric oxide production, but is correlated with an enhancement of fungicidal activity of cytoplasmic cationic proteases. We speculate that encapsulation of C. neoformans could down-regulate the development of the immune response mediated by Cryptococcus-laden AM at lung level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolaños B., Mitchell T. G. Phagocytosis of Cryptococcus neoformans by rat alveolar macrophages. J Med Vet Mycol. 1989;27(4):203–217. [PubMed] [Google Scholar]
- Clumeck N., Hermans P., De Wit S. Current problems in the management of AIDS patients. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):2–10. doi: 10.1007/BF01962163. [DOI] [PubMed] [Google Scholar]
- Collins H. L., Bancroft G. J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992 Jun;22(6):1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- Collins H. L., Bancroft G. J. Encapsulation of Cryptococcus neoformans impairs antigen-specific T-cell responses. Infect Immun. 1991 Nov;59(11):3883–3888. doi: 10.1128/iai.59.11.3883-3888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Fromtling R. A., Shadomy H. J. An overview of macrophage-fungal interactions. Mycopathologia. 1986 Feb;93(2):77–93. doi: 10.1007/BF00437738. [DOI] [PubMed] [Google Scholar]
- Good C. B., Coax W. A. Cryptococcal infections in patients with AIDS. N Engl J Med. 1990 Mar 8;322(10):701–702. doi: 10.1056/nejm199003083221017. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Hidore M. R., Mislan T. W., Murphy J. W. Responses of murine natural killer cells to binding of the fungal target Cryptococcus neoformans. Infect Immun. 1991 Apr;59(4):1489–1499. doi: 10.1128/iai.59.4.1489-1499.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Sonleitner F., Murphy J. W. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991 May;59(5):1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992 Jun 1;175(6):1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Dunn P. L. A T cell-independent protective host response against Cryptococcus neoformans expressed at the primary site of infection in the lung. Infect Immun. 1993 Dec;61(12):5302–5308. doi: 10.1128/iai.61.12.5302-5308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Harmsen A. G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991 Mar 1;173(3):755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaoui R. M., Hall N. K., Larsh H. W. Role of macrophages in immunity and pathogenesis of experimental cryptococcosis induced by the airborne route--Part II: Phagocytosis and intracellular fate of Cryptococcus neoformans. Mykosen. 1977 Nov;20(11):409–412. [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Redelman D. Activated neutrophils exhibit enhanced phagocytosis of Cryptococcus neoformans opsonized with normal human serum. Clin Exp Immunol. 1987 Oct;70(1):238–246. [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Dupont M. P. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J Clin Invest. 1993 Apr;91(4):1490–1498. doi: 10.1172/JCI116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991 Jan;59(1):24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991 Jan;59(1):24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Syme R. M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993 Feb;61(2):464–469. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Tyler C. L., Sitrin R. G., Jackson C., Toews G. B. Interferon-gamma activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991 Jul;5(1):19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- Murphy J. W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993 Nov;61(11):4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992 Oct;146(4):1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Dottorini M., Beccari T., Cociani C., Todisco T., Bistoni F. Inhibition of candidacidal activity of polymorphonuclear cells by alveolar macrophage-derived factor from lung cancer patients. Am Rev Respir Dis. 1993 Feb;147(2):414–419. doi: 10.1164/ajrccm/147.2.414. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Dottorini M., Cociani C., Pietrella D., Todisco T., Bistoni F. Mechanism of intracellular candidacidal activity mediated by calcium ionophore in human alveolar macrophages. Am J Respir Cell Mol Biol. 1993 Jul;9(1):19–25. doi: 10.1165/ajrcmb/9.1.19. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Dottorini M., Pietrella D., Cociani C., Eslami A., Todisco T., Bistoni F. Macrophage activation by N-acetyl-cysteine in COPD patients. Chest. 1994 Mar;105(3):806–811. doi: 10.1378/chest.105.3.806. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Dottorini M., Pietrella D., Monari C., Retini C., Todisco T., Bistoni F. Role of human alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994 Aug;11(2):130–137. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- Weinberg P. B., Becker S., Granger D. L., Koren H. S. Growth inhibition of Cryptococcus neoformans by human alveolar macrophages. Am Rev Respir Dis. 1987 Nov;136(5):1242–1247. doi: 10.1164/ajrccm/136.5.1242. [DOI] [PubMed] [Google Scholar]


