Abstract
As elevated levels of glycated IgG have been detected in the plasma of patients with diabetes mellitus, a disease associated with increased susceptibility to infection, we have investigated whether glycation of MoAbs affects the kinetics and/or affinity of antigen binding. Three mouse MoAbs were incubated with 0.5 M glucose at pH 7.4 for 14-21 days at 37 degrees C. Control MoAbs were incubated using identical conditions but with no added glucose. Using a surface plasmon resonance technique we found that glycation significantly increased the rate of dissociation (kdiss) of the antigen-antibody complex for all three MoAbs (P < 0.05, n = 4), but had no significant effect on the rate of association (kass). For one of the MoAbs, against human IgG (Fab), we also measured kdiss by an alternative method utilizing radiolabelled antigen, which confirmed that glycation of the antibody significantly increases kdiss (P < 0.001, n = 8). We also found using an ELISA-based method that glycation of the same MoAb significantly increased the equilibrium dissociation constant (Kd) (P < 0.05, n = 6). A significant increase in kd was observed after glycation using glucose concentrations consistent with those found in poorly controlled diabetics (P < 0.02, n = 5). We conclude that in vitro glycation can significantly lower the affinity of an antibody for its antigen, and significantly increases the rate of dissociation of the antigen-antibody complex.
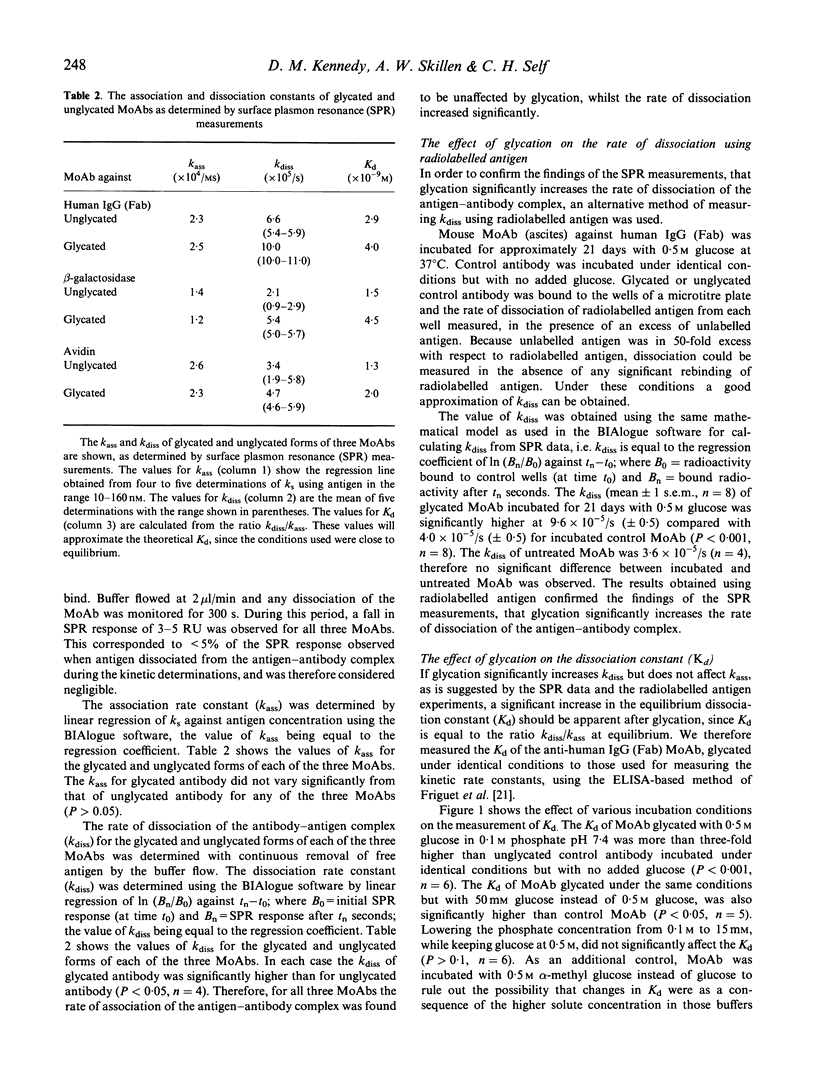
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baynes J. W., Thorpe S. R., Murtiashaw M. H. Nonenzymatic glucosylation of lysine residues in albumin. Methods Enzymol. 1984;106:88–98. doi: 10.1016/0076-6879(84)06010-9. [DOI] [PubMed] [Google Scholar]
- Bissé E., Berger W., Flückiger R. Quantitation of glycosylated hemoglobin. Elimination of labile glycohemoglobin during sample hemolysis at pH 5. Diabetes. 1982 Jul;31(7):630–633. doi: 10.2337/diab.31.7.630. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992 Dec;15(12):1835–1843. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Shapiro R., McManus M., Garrick L., McDonald M. J., Gallop P. M., Gabbay K. H. Structural heterogeneity of human hemoglobin A due to nonenzymatic glycosylation. J Biol Chem. 1979 May 25;254(10):3892–3898. [PubMed] [Google Scholar]
- Danze P. M., Tarjoman A., Rousseaux J., Fossati P., Dautrevaux M. Evidence for an increased glycation of IgG in diabetic patients. Clin Chim Acta. 1987 Jul 15;166(2-3):143–153. doi: 10.1016/0009-8981(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Dolhofer-Bliesener R., Gerbitz K. D. Impairment by glycation of immunoglobulin G Fc fragment function. Scand J Clin Lab Invest. 1990 Nov;50(7):739–746. doi: 10.3109/00365519009091067. [DOI] [PubMed] [Google Scholar]
- Dolhofer R., Siess E. A., Wieland O. H. Nonenzymatic glycation of immunoglobulins leads to an impairment of immunoreactivity. Biol Chem Hoppe Seyler. 1985 Apr;366(4):361–366. doi: 10.1515/bchm3.1985.366.1.361. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Gould B. J., Hall P. M., Cook J. G. A sensitive method for the measurement of glycosylated plasma proteins using affinity chromatography. Ann Clin Biochem. 1984 Jan;21(Pt 1):16–21. doi: 10.1177/000456328402100103. [DOI] [PubMed] [Google Scholar]
- Hammes H. P., Kiefel V., Laube H., Federlin K. Impaired agglutination of IgM resulting from non-enzymatic glycation in diabetes mellitus. Diabetes Res Clin Pract. 1990 Apr;9(1):37–42. doi: 10.1016/0168-8227(90)90006-f. [DOI] [PubMed] [Google Scholar]
- Kaneshige H. Nonenzymatic glycosylation of serum IgG and its effect on antibody activity in patients with diabetes mellitus. Diabetes. 1987 Jul;36(7):822–828. doi: 10.2337/diab.36.7.822. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991 Dec 15;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Kennedy D. M., Skillen A. W., Self C. H. Colorimetric assay of glycoprotein glycation free of interference from glycosylation residues. Clin Chem. 1993 Nov;39(11 Pt 1):2309–2311. [PubMed] [Google Scholar]
- Kennedy L., Baynes J. W. Non-enzymatic glycosylation and the chronic complications of diabetes: an overview. Diabetologia. 1984 Feb;26(2):93–98. doi: 10.1007/BF00281113. [DOI] [PubMed] [Google Scholar]
- Larkin J. G., Frier B. M., Ireland J. T. Diabetes mellitus and infection. Postgrad Med J. 1985 Mar;61(713):233–237. doi: 10.1136/pgmj.61.713.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist M. Surface plasmon resonance for detection and measurement of antibody-antigen affinity and kinetics. Curr Opin Immunol. 1993 Apr;5(2):282–286. doi: 10.1016/0952-7915(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Morin L. G., Austin G. E., Burkhalter A. Nonenzymatic glycation of immunoglobulins does not impair antigen-antibody binding. Clin Chem. 1987 May;33(5):692–694. [PubMed] [Google Scholar]
- Morin L. G., Austin G. E., Rodey G. E., Johnson J. E. Nonenzymic glycation of human immunoglobulins does not impair their immunoreactivity. Clin Chem. 1989 Jun;35(6):1039–1042. [PubMed] [Google Scholar]
- Moutschen M. P., Scheen A. J., Lefebvre P. J. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992 May-Jun;18(3):187–201. [PubMed] [Google Scholar]
- Rayfield E. J., Ault M. J., Keusch G. T., Brothers M. J., Nechemias C., Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982 Mar;72(3):439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Mori T., Shiiki H., Dohi K., Ishikawa H. Non-enzymatic glycosylation of mouse monoclonal antibody reduces its binding activity to antigen. Clin Chim Acta. 1993 Oct 29;220(1):119–121. doi: 10.1016/0009-8981(93)90013-t. [DOI] [PubMed] [Google Scholar]
- Stanley C., Lew A. M., Steward M. W. The measurement of antibody affinity: a comparison of five techniques utilizing a panel of monoclonal anti-DNP antibodies and the effect of high affinity antibody on the measurement of low affinity antibody. J Immunol Methods. 1983 Nov 11;64(1-2):119–132. doi: 10.1016/0022-1759(83)90390-3. [DOI] [PubMed] [Google Scholar]
- Stevens F. J. Modification of an ELISA-based procedure for affinity determination: correction necessary for use with bivalent antibody. Mol Immunol. 1987 Oct;24(10):1055–1060. doi: 10.1016/0161-5890(87)90073-3. [DOI] [PubMed] [Google Scholar]


