Abstract
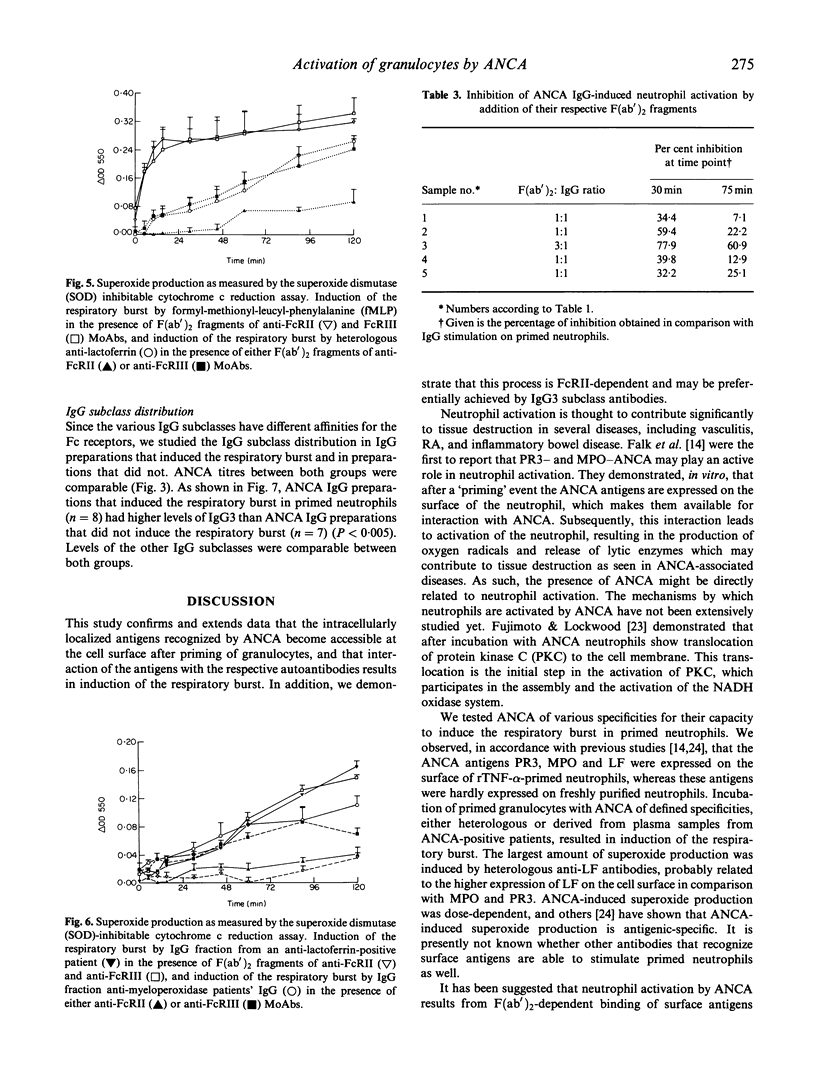
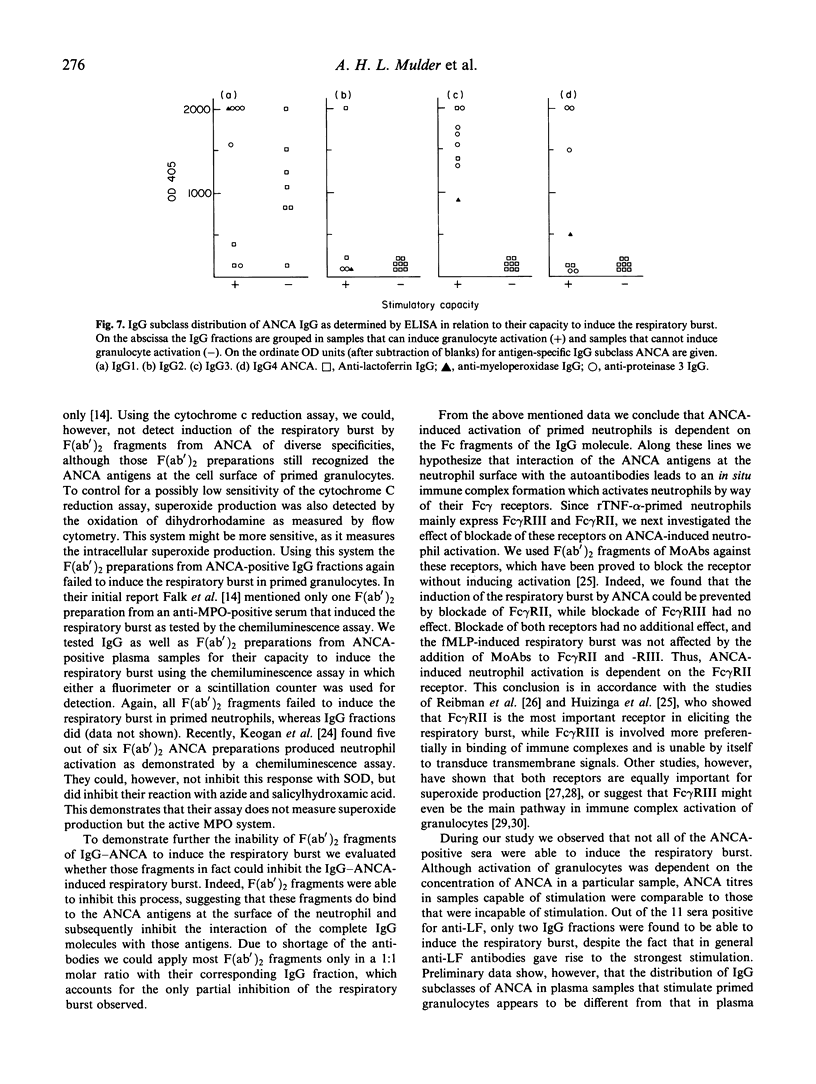
ANCA have been demonstrated to induce the respiratory burst in primed neutrophils. In this study we have extended the investigations on neutrophil activation by ANCA directed against proteinase 3 (PR3), myeloperoxidase (MPO) and lactoferrin (LF), and we have analysed the underlying mechanisms. All three ANCA antigens were expressed on the cell surface of primed neutrophils. Superoxide production assayed by both cytochrome c reduction and oxidation of dihydrorhodamine 123, was induced by heterologous polyclonal anti-MPO and anti-LF antibodies, and ANCA-positive plasma samples. Induction of superoxide production was dose-dependent. F(ab')2 fragments did not induce the respiratory burst. Blockade of Fc receptors by specific MoAbs showed that anti-Fc gamma RII antibodies were able to turn off the ANCA-induced respiratory burst, whereas anti-Fc gamma RIII antibodies did not. Plasma samples that induced the respiratory burst did not differ from samples that did not induce superoxide production with respect to ANCA titre, but had higher levels of the IgG3 subclass of ANCA. Levels of the other subclasses of ANCA were comparable between those samples. We conclude that ANCA-induced activation of primed neutrophils is Fc gamma RII-dependent, and appears to be facilitated by antibodies of the IgG3 subclass.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Shen L., Eicher D. M., Wewers M. D., Gill J. K. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med. 1990 Apr 1;171(4):1333–1345. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer E., Tervaert J. W., Horst G., Huitema M. G., van der Giessen M., Limburg P. C., Kallenberg C. G. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener's granulomatosis and clinically related disorders. Clin Exp Immunol. 1991 Mar;83(3):379–386. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkhorst B. A., Strohmeier G., Lazzari K., Weil G., Melnick D., Fleit H. B., Simons E. R. Differential roles of Fc gamma RII and Fc gamma RIII in immune complex stimulation of human neutrophils. J Biol Chem. 1992 Oct 15;267(29):20659–20666. [PubMed] [Google Scholar]
- Coremans I. E., Hagen E. C., Daha M. R., van der Woude F. J., van der Voort E. A., Kleijburg-van der Keur C., Breedveld F. C. Antilactoferrin antibodies in patients with rheumatoid arthritis are associated with vasculitis. Arthritis Rheum. 1992 Dec;35(12):1466–1475. doi: 10.1002/art.1780351210. [DOI] [PubMed] [Google Scholar]
- Crockett-Torabi E., Fantone J. C. Soluble and insoluble immune complexes activate human neutrophil NADPH oxidase by distinct Fc gamma receptor-specific mechanisms. J Immunol. 1990 Nov 1;145(9):3026–3032. [PubMed] [Google Scholar]
- Ewert B. H., Jennette J. C., Falk R. J. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992 Feb;41(2):375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988 Jun 23;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Terrell R. S., Charles L. A., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., Kerst M., Nuyens J. H., Vlug A., von dem Borne A. E., Roos D., Tetteroo P. A. Binding characteristics of dimeric IgG subclass complexes to human neutrophils. J Immunol. 1989 Apr 1;142(7):2359–2364. [PubMed] [Google Scholar]
- Huizinga T. W., van Kemenade F., Koenderman L., Dolman K. M., von dem Borne A. E., Tetteroo P. A., Roos D. The 40-kDa Fc gamma receptor (FcRII) on human neutrophils is essential for the IgG-induced respiratory burst and IgG-induced phagocytosis. J Immunol. 1989 Apr 1;142(7):2365–2369. [PubMed] [Google Scholar]
- Hundt M., Schmidt R. E. The glycosylphosphatidylinositol-linked Fc gamma receptor III represents the dominant receptor structure for immune complex activation of neutrophils. Eur J Immunol. 1992 Mar;22(3):811–816. doi: 10.1002/eji.1830220327. [DOI] [PubMed] [Google Scholar]
- Jennette J. C., Charles L. A., Falk R. J. Antineutrophil cytoplasmic autoantibodies: disease associations, molecular biology, and pathophysiology. Int Rev Exp Pathol. 1991;32:193–221. doi: 10.1016/b978-0-12-364932-4.50009-5. [DOI] [PubMed] [Google Scholar]
- Kallenberg C. G., Mulder A. H., Tervaert J. W. Antineutrophil cytoplasmic antibodies: a still-growing class of autoantibodies in inflammatory disorders. Am J Med. 1992 Dec;93(6):675–682. doi: 10.1016/0002-9343(92)90202-m. [DOI] [PubMed] [Google Scholar]
- Keogan M. T., Esnault V. L., Green A. J., Lockwood C. M., Brown D. L. Activation of normal neutrophils by anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1992 Nov;90(2):228–234. doi: 10.1111/j.1365-2249.1992.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder A. H., Broekroelofs J., Horst G., Limburg P. C., Nelis G. F., Kallenberg C. G. Anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel disease: characterization and clinical correlates. Clin Exp Immunol. 1994 Mar;95(3):490–497. doi: 10.1111/j.1365-2249.1994.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder A. H., Horst G., Haagsma E. B., Limburg P. C., Kleibeuker J. H., Kallenberg C. G. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993 Mar;17(3):411–417. [PubMed] [Google Scholar]
- Mulder A. H., Horst G., van Leeuwen M. A., Limburg P. C., Kallenberg C. G. Antineutrophil cytoplasmic antibodies in rheumatoid arthritis. Characterization and clinical correlations. Arthritis Rheum. 1993 Aug;36(8):1054–1060. doi: 10.1002/art.1780360805. [DOI] [PubMed] [Google Scholar]
- Peen E., Almer S., Bodemar G., Rydén B. O., Sjölin C., Tejle K., Skogh T. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993 Jan;34(1):56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibman J., Haines K. A., Gude D., Weissmann G. Differences in signal transduction between Fc gamma receptors (Fc gamma RII, Fc gamma RIII) and FMLP receptors in neutrophils. Effects of colchicine on pertussis toxin sensitivity and diacylglycerol formation. J Immunol. 1991 Feb 1;146(3):988–996. [PubMed] [Google Scholar]
- Savage C. O., Gaskin G., Pusey C. D., Pearson J. D. Anti-neutrophil cytoplasm antibodies can recognize vascular endothelial cell-bound anti-neutrophil cytoplasm antibody-associated autoantigens. Exp Nephrol. 1993 May-Jun;1(3):190–195. [PubMed] [Google Scholar]
- Saxon A., Shanahan F., Landers C., Ganz T., Targan S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990 Aug;86(2):202–210. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Goldschmeding R., Elema J. D., Limburg P. C., van der Giessen M., Huitema M. G., Koolen M. I., Hené R. J., The T. H., van der Hem G. K. Association of autoantibodies to myeloperoxidase with different forms of vasculitis. Arthritis Rheum. 1990 Aug;33(8):1264–1272. doi: 10.1002/art.1780330829. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Goldschmeding R., Elema J. D., van der Giessen M., Huitema M. G., van der Hem G. K., The T. H., von dem Borne A. E., Kallenberg C. G. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 1990 Feb;37(2):799–806. doi: 10.1038/ki.1990.48. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Huitema M. G., Hené R. J., Sluiter W. J., The T. H., van der Hem G. K., Kallenberg C. G. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990 Sep 22;336(8717):709–711. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., van der Woude F. J., Fauci A. S., Ambrus J. L., Velosa J., Keane W. F., Meijer S., van der Giessen M., van der Hem G. K., The T. H. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989 Nov;149(11):2461–2465. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- van der Woude F. J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L. A., van der Giessen M., van der Hem G. K., The T. H. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985 Feb 23;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]


