Abstract
The targeting and recruitment of inflammatory cells to vascular endothelium in Graves' disease (GD) is mediated by intercellular adhesion molecule-1 (ICAM-1), endothelial leucocyte adhesion molecule-1 (ELAM-1), and vascular cell adhesion molecule-1 (VCAM-1). We have studied serum levels of soluble ICAM-1 (sICAM-1), soluble ELAM-1 (sELAM-1), and soluble VCAM-1 (sVCAM-1) in patients with GD (n = 21) and in patients with iodine-deficient goitre (IDG) (n = 23). The serum levels of sICAM-1 were markedly elevated in patients with GD before treatment with thiamazole (median 560 ng/ml versus 185 ng/ml in patients with IDG). In addition, elevated serum concentrations of sELAM-1 (median 85 ng/ml versus 33 ng/ml, respectively) and sVCAM-1 (median 42 ng/ml versus 15 ng/ml, respectively) were observed in patients with GD (P < 0.01 for all). The serum levels of sELAM-1 and sVCAM-1 dropped significantly after initiation of therapy and were within the normal range after 4, and 8 weeks of therapy, respectively. Serum levels of sICAM-1 were elevated even after 8 weeks of therapy. Serum levels of sVACM-1 and sICAM-1 correlated with the serum concentrations of anti-thyroid-stimulating hormone (TSH)-receptor antibodies (TSHR-R) (n = 21; r = 0.929 and r = 0.810, respectively) and anti-thyroid peroxidase antibodies (TPO-Ab) (n = 21; r = 0.673 and r = 0.750, respectively). However, no correlation between sELAM-1 and TPO-Ab, TSHR-R, and anti-thyroglobulin antibodies (Tg-Ab), respectively, could be found. In addition to thyroid hormones and autoantibodies, serum concentrations of sELAM-1 and sVCAM-1, but not sICAM-1, could be useful as clinical markers for disease activity.
Full text
PDF
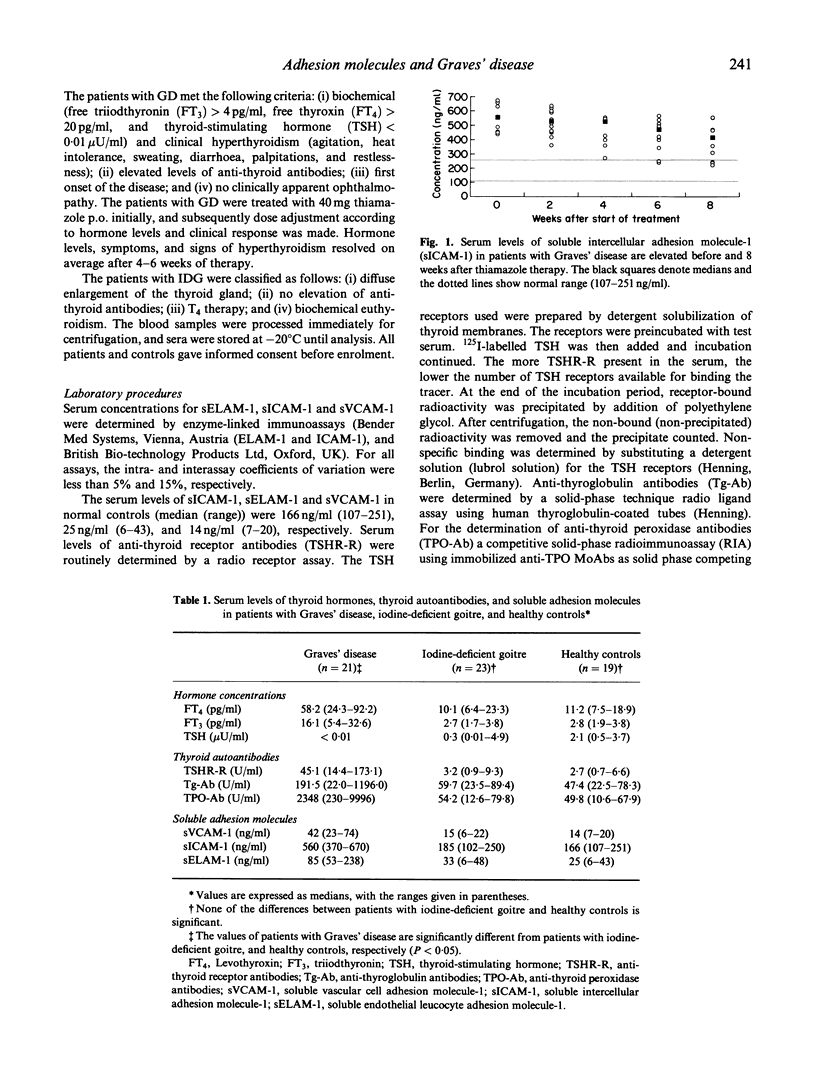
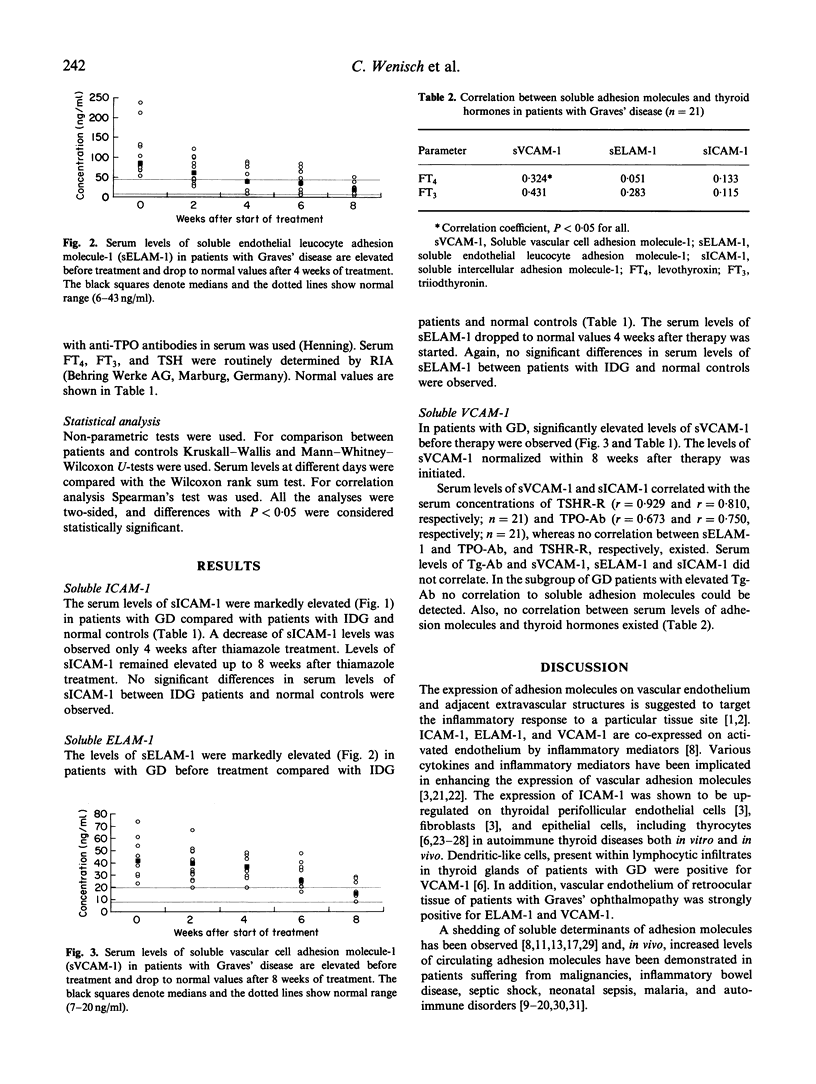


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991 Mar;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- Bagnasco M., Caretto A., Olive D., Pedini B., Canonica G. W., Betterle C. Expression of intercellular adhesion molecule-1 (ICAM-1) on thyroid epithelial cells in Hashimoto's thyroiditis but not in Graves' disease or papillary thyroid cancer. Clin Exp Immunol. 1991 Feb;83(2):309–313. doi: 10.1111/j.1365-2249.1991.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks R. E., Gearing A. J., Hemingway I. K., Norfolk D. R., Perren T. J., Selby P. J. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993 Jul;68(1):122–124. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. C., Dummer R., Hartmann A. A., Burg G., Schmidt R. E. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. Functional consequences on cell-mediated cytotoxicity. J Immunol. 1991 Dec 15;147(12):4398–4401. [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Fowler P. D., Tacker M., Whitley G. S., Meager A., Nussey S. S., Johnstone A. P. Expression of intercellular adhesion molecule-1 (ICAM-1) on human thyroid cells lines correlated with their binding of lymphoblasts. Mol Cell Endocrinol. 1990 May 28;71(1):55–61. doi: 10.1016/0303-7207(90)90075-j. [DOI] [PubMed] [Google Scholar]
- Fukazawa H., Yoshida K., Ichinohasama R., Sawai T., Hiromatsu Y., Mori K., Kikuchi K., Aizawa Y., Abe K., Wall J. R. Expression of the Hermes-1 (CD44) and ICAM-1 (CD54) molecule on the surface of thyroid cells from patients with Graves' disease. Thyroid. 1993 Winter;3(4):285–289. doi: 10.1089/thy.1993.3.285. [DOI] [PubMed] [Google Scholar]
- Furukawa S., Imai K., Matsubara T., Yone K., Yachi A., Okumura K., Yabuta K. Increased levels of circulating intercellular adhesion molecule 1 in Kawasaki disease. Arthritis Rheum. 1992 Jun;35(6):672–677. doi: 10.1002/art.1780350611. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Hemingway I., Pigott R., Hughes J., Rees A. J., Cashman S. J. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann N Y Acad Sci. 1992 Dec 4;667:324–331. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- Graninger W., Pirich K. R., Speiser W., Deutsch E., Waldhäusl W. K. Effect of thyroid hormones on plasma protein concentrations in man. J Clin Endocrinol Metab. 1986 Aug;63(2):407–411. doi: 10.1210/jcem-63-2-407. [DOI] [PubMed] [Google Scholar]
- Harning R., Mainolfi E., Bystryn J. C., Henn M., Merluzzi V. J., Rothlein R. Serum levels of circulating intercellular adhesion molecule 1 in human malignant melanoma. Cancer Res. 1991 Sep 15;51(18):5003–5005. [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Elevated expression in situ of selectin and immunoglobulin superfamily type adhesion molecules in retroocular connective tissues from patients with Graves' ophthalmopathy. Clin Exp Immunol. 1993 Mar;91(3):381–389. doi: 10.1111/j.1365-2249.1993.tb05913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Graves' immunoglobulins and cytokines stimulate the expression of intercellular adhesion molecule-1 (ICAM-1) in cultured Graves' orbital fibroblasts. Eur J Clin Invest. 1992 Aug;22(8):529–537. doi: 10.1111/j.1365-2362.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Graves' immunoglobulins and cytokines stimulate the expression of intercellular adhesion molecule-1 (ICAM-1) in cultured Graves' orbital fibroblasts. Eur J Clin Invest. 1992 Aug;22(8):529–537. doi: 10.1111/j.1365-2362.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Soluble intercellular adhesion molecule-1 (sICAM-1) in sera of patients with Graves' ophthalmopathy and thyroid diseases. Clin Exp Immunol. 1993 May;92(2):296–302. doi: 10.1111/j.1365-2249.1993.tb03395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L., Theander T. G., Elhassan I. M., Jensen J. B. Increased plasma levels of soluble ICAM-1 and ELAM-1 (E-selectin) during acute Plasmodium falciparum malaria. Immunol Lett. 1993 Apr;36(1):51–58. doi: 10.1016/0165-2478(93)90068-d. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Küster H., Degitz K. Circulating ICAM-1 in neonatal sepsis. Lancet. 1993 Feb 20;341(8843):506–506. doi: 10.1016/0140-6736(93)90272-i. [DOI] [PubMed] [Google Scholar]
- Lampeter E. R., Kishimoto T. K., Rothlein R., Mainolfi E. A., Bertrams J., Kolb H., Martin S. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992 Dec;41(12):1668–1671. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Bernard A., Sanders M. E. Cell adhesion/signalling: biology and clinical applications. Eur J Clin Invest. 1992 Jul;22(7):443–453. doi: 10.1111/j.1365-2362.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Martin A., Huber G. K., Davies T. F. Induction of human thyroid cell ICAM-1 (CD54) antigen expression and ICAM-1-mediated lymphocyte binding. Endocrinology. 1990 Aug;127(2):651–657. doi: 10.1210/endo-127-2-651. [DOI] [PubMed] [Google Scholar]
- Miyazaki A., Mirakian R., Bottazzo G. F. Adhesion molecule expression in Graves' thyroid glands; potential relevance of granule membrane protein (GMP-140) and intercellular adhesion molecule-1 (ICAM-1) in the homing and antigen presentation processes. Clin Exp Immunol. 1992 Jul;89(1):52–57. doi: 10.1111/j.1365-2249.1992.tb06876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman W., Beall L. D., Carson C. W., Hunder G. G., Graben N., Randhawa Z. I., Gopal T. V., Wiener-Kronish J., Matthay M. A. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol. 1993 Jan 15;150(2):644–654. [PubMed] [Google Scholar]
- Norris P., Poston R. N., Thomas D. S., Thornhill M., Hawk J., Haskard D. O. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991 May;96(5):763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- Paschke R., Vogg M., Swillens S., Usadel K. H. Correlation of microsomal antibodies with the intensity of the intrathyroidal autoimmune process in Graves' disease. J Clin Endocrinol Metab. 1993 Oct;77(4):939–943. doi: 10.1210/jcem.77.4.8408468. [DOI] [PubMed] [Google Scholar]
- Pigott R., Dillon L. P., Hemingway I. H., Gearing A. J. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992 Sep 16;187(2):584–589. doi: 10.1016/0006-291x(92)91234-h. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Shijubo N., Imai K., Aoki S., Hirasawa M., Sugawara H., Koba H., Tsujisaki M., Sugiyama T., Hinoda Y., Yachi A. Circulating intercellular adhesion molecule-1 (ICAM-1) antigen in sera of patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1992 Jul;89(1):58–62. doi: 10.1111/j.1365-2249.1992.tb06877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Ockenhouse C. F., Springer T. A. Soluble intercellular adhesion molecule 1-immunoglobulin G1 immunoadhesin mediates phagocytosis of malaria-infected erythrocytes. J Exp Med. 1992 Nov 1;176(5):1471–1476. doi: 10.1084/jem.176.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa E., Roura C., Catálfamo M., Martí M., Lucas-Martín A., Sanmartí A., Salinas I., Obiols G., Foz-Sala M., Pujol-Borrell R. Expression of intercellular adhesion molecule-1 in thyroid follicular cells in autoimmune, non-autoimmune and neoplastic diseases of the thyroid gland: discordance with HLA. J Autoimmun. 1992 Feb;5(1):107–118. doi: 10.1016/s0896-8411(05)80055-1. [DOI] [PubMed] [Google Scholar]
- Tsujisaki M., Imai K., Hirata H., Hanzawa Y., Masuya J., Nakano T., Sugiyama T., Matsui M., Hinoda Y., Yachi A. Detection of circulating intercellular adhesion molecule-1 antigen in malignant diseases. Clin Exp Immunol. 1991 Jul;85(1):3–8. doi: 10.1111/j.1365-2249.1991.tb05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Makgoba M. W., Borysiewicz L. K. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J Endocrinol. 1989 Jul;122(1):185–191. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]
- Wenisch C., Looareesuwan S., Parschalk B., Graninger W. Soluble vascular cell adhesion molecule 1 is elevated in patients with Plasmodium falciparum malaria. J Infect Dis. 1994 Mar;169(3):710–711. doi: 10.1093/infdis/169.3.710. [DOI] [PubMed] [Google Scholar]
- Zheng R. Q., Abney E. R., Grubeck-Loebenstein B., Dayan C., Maini R. N., Feldmann M. Expression of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-3 on human thyroid epithelial cells in Graves' and Hashimoto's diseases. J Autoimmun. 1990 Dec;3(6):727–736. doi: 10.1016/s0896-8411(05)80039-3. [DOI] [PubMed] [Google Scholar]


