Abstract
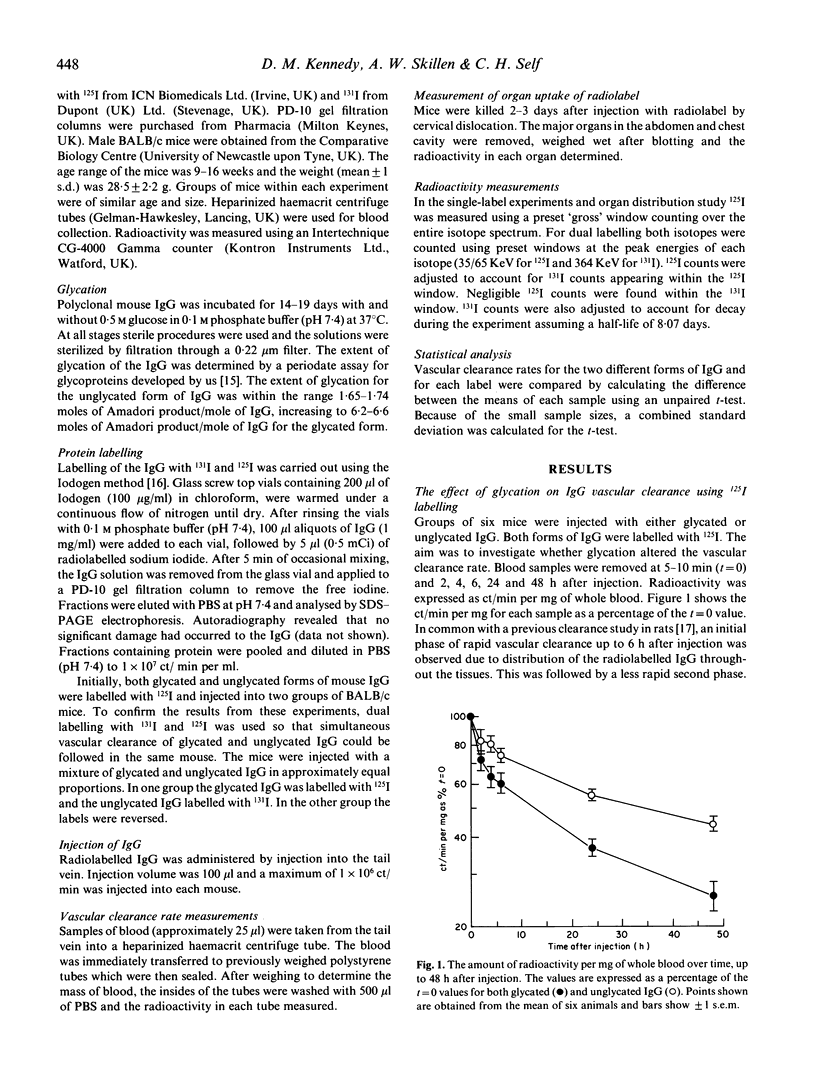
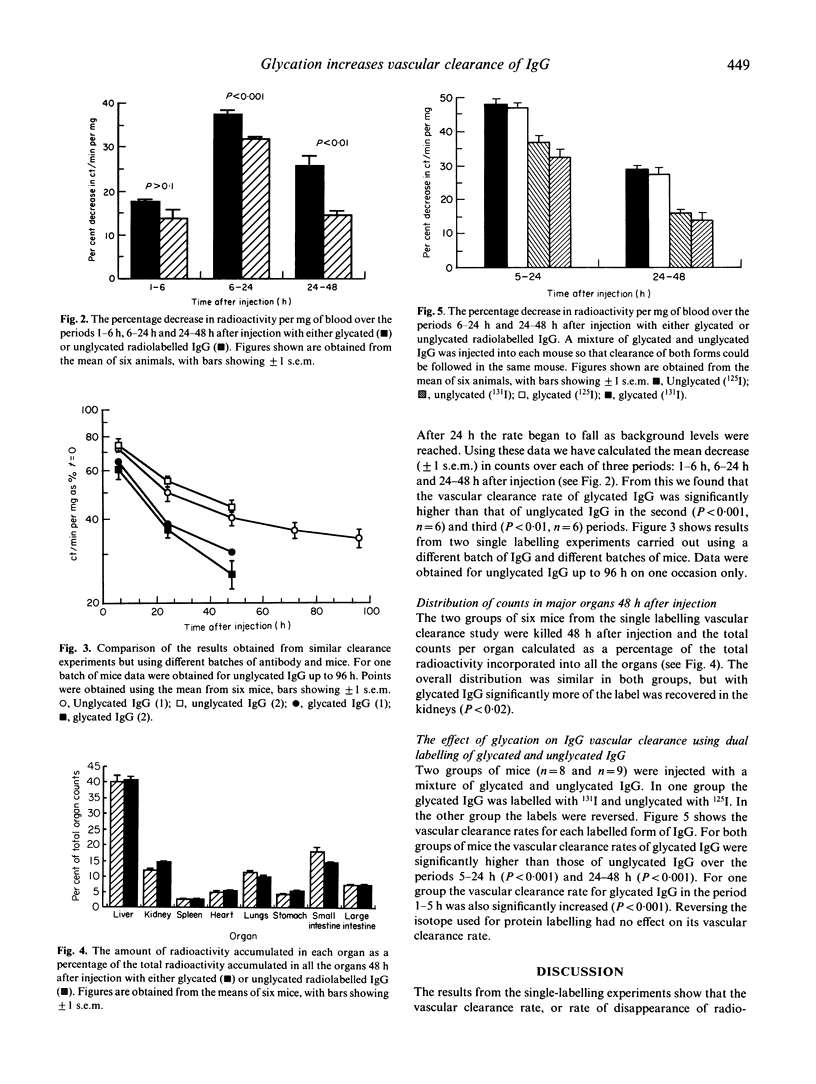
As elevated levels of glycated IgG have been detected in the plasma of diabetics we have investigated whether glycation of IgG affects its vascular clearance rate, using a mouse model system. Polyclonal mouse IgG was aseptically incubated for 14-19 days with 0.5 M glucose in 0.1 M phosphate buffer (pH 7.4) at 37 degrees C. As control, IgG was incubated under identical conditions but with no added glucose. After incubation, both forms were labelled with 125I and injected intravenously into BALB/c mice. The rate of vascular clearance of the glycated IgG was found to be significantly higher than the control IgG in the periods 5-24 h (P < 0.001, n = 6) and 24-48 h (P < 0.01, n = 6) after injection. After 2-3 days the mice were killed and the major organs were harvested. With glycated IgG there was a significant increase in the 125I accumulated in the kidney (P < 0.02). In later experiments, dual labelling with 131I and 125I allowed mixtures of glycated and unglycated IgG to be injected into the same mouse so that the vascular clearance of both forms of IgG could be followed simultaneously. These experiments confirmed that glycation of the IgG significantly increases its vascular clearance rate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baynes J. W., Thorpe S. R., Murtiashaw M. H. Nonenzymatic glucosylation of lysine residues in albumin. Methods Enzymol. 1984;106:88–98. doi: 10.1016/0076-6879(84)06010-9. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Watkins N. G., Fisher C. I., Hull C. J., Patrick J. S., Ahmed M. U., Dunn J. A., Thorpe S. R. The Amadori product on protein: structure and reactions. Prog Clin Biol Res. 1989;304:43–67. [PubMed] [Google Scholar]
- Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992 Dec;15(12):1835–1843. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Pongor S., Cerami A. Covalent attachment of soluble proteins by nonenzymatically glycosylated collagen. Role in the in situ formation of immune complexes. J Exp Med. 1983 Nov 1;158(5):1739–1744. doi: 10.1084/jem.158.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danze P. M., Tarjoman A., Rousseaux J., Fossati P., Dautrevaux M. Evidence for an increased glycation of IgG in diabetic patients. Clin Chim Acta. 1987 Jul 15;166(2-3):143–153. doi: 10.1016/0009-8981(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Dolhofer-Bliesener R., Gerbitz K. D. Effect of nonenzymatic glycation on the structure of immunoglobulin G. Biol Chem Hoppe Seyler. 1990 Aug;371(8):693–697. doi: 10.1515/bchm3.1990.371.2.693. [DOI] [PubMed] [Google Scholar]
- Dolhofer-Bliesener R., Gerbitz K. D. Impairment by glycation of immunoglobulin G Fc fragment function. Scand J Clin Lab Invest. 1990 Nov;50(7):739–746. doi: 10.3109/00365519009091067. [DOI] [PubMed] [Google Scholar]
- Dolhofer R., Siess E. A., Wieland O. H. Nonenzymatic glycation of immunoglobulins leads to an impairment of immunoreactivity. Biol Chem Hoppe Seyler. 1985 Apr;366(4):361–366. doi: 10.1515/bchm3.1985.366.1.361. [DOI] [PubMed] [Google Scholar]
- Eccles S. A., Purvies H. P., Styles J. M., Hobbs S. M., Dean C. J. Pharmacokinetic studies of radiolabelled rat monoclonal antibodies recognising syngeneic sarcoma antigens. I. Comparison of IgG subclasses. Cancer Immunol Immunother. 1989;30(1):5–12. doi: 10.1007/BF01665024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hammes H. P., Kiefel V., Laube H., Federlin K. Impaired agglutination of IgM resulting from non-enzymatic glycation in diabetes mellitus. Diabetes Res Clin Pract. 1990 Apr;9(1):37–42. doi: 10.1016/0168-8227(90)90006-f. [DOI] [PubMed] [Google Scholar]
- Kaneshige H. Nonenzymatic glycosylation of serum IgG and its effect on antibody activity in patients with diabetes mellitus. Diabetes. 1987 Jul;36(7):822–828. doi: 10.2337/diab.36.7.822. [DOI] [PubMed] [Google Scholar]
- Larkin J. G., Frier B. M., Ireland J. T. Diabetes mellitus and infection. Postgrad Med J. 1985 Mar;61(713):233–237. doi: 10.1136/pgmj.61.713.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L. G., Austin G. E., Burkhalter A. Nonenzymatic glycation of immunoglobulins does not impair antigen-antibody binding. Clin Chem. 1987 May;33(5):692–694. [PubMed] [Google Scholar]
- Pollock R. R., French D. L., Metlay J. P., Birshtein B. K., Scharff M. D. Intravascular metabolism of normal and mutant mouse immunoglobulin molecules. Eur J Immunol. 1990 Sep;20(9):2021–2027. doi: 10.1002/eji.1830200921. [DOI] [PubMed] [Google Scholar]
- Rahbar S., Blumenfeld O., Ranney H. M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969 Aug 22;36(5):838–843. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- Rayfield E. J., Ault M. J., Keusch G. T., Brothers M. J., Nechemias C., Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982 Mar;72(3):439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]



