Abstract
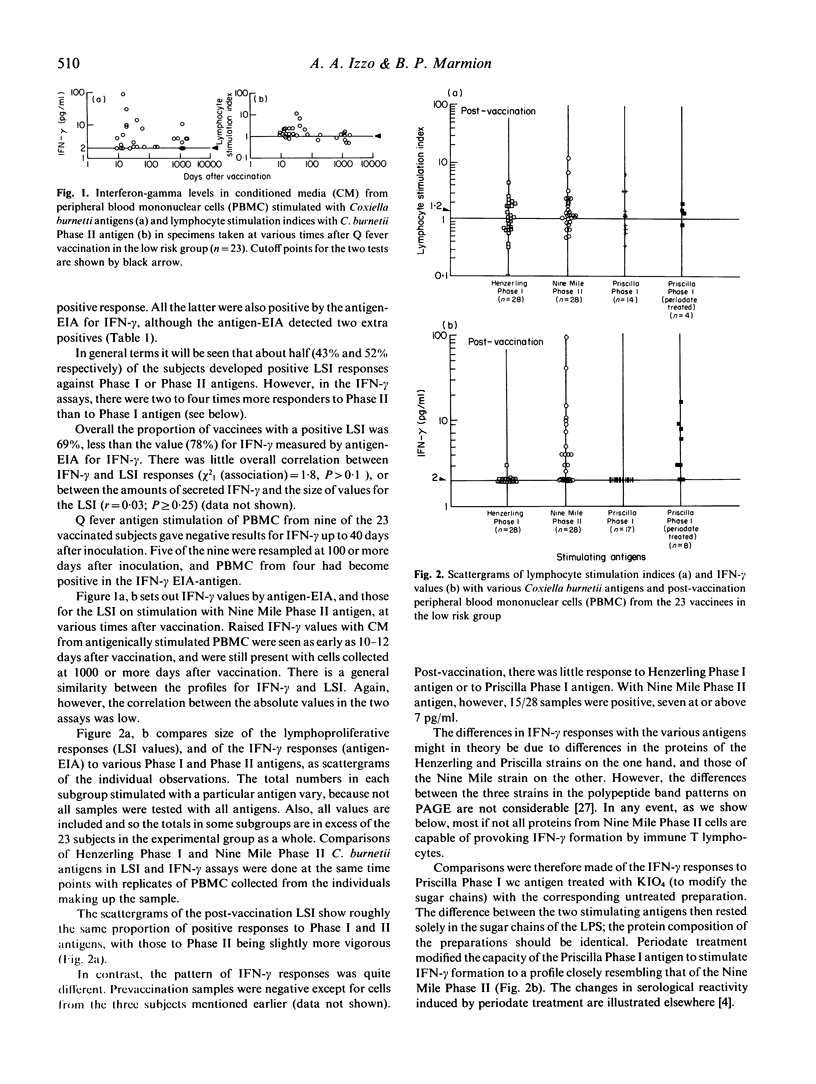
Previous work in our laboratory has shown that lymphocytes from persons vaccinated with a formalin-inactivated Phase I Q fever vaccine (Q-Vax CSL Ltd) show a mitogenic response to Coxiella burnetii antigens. The mitogenic response is the sum of that from various subsets of CD4+, T helper cells, CD8+ T cells and probably B cells. It does not distinguish between T helper cell responses leading to formation of interferon-gamma (IFN-gamma)--a cytokine responsible for clearing intracellular infection with C. burnetii organisms--and responses of other T cell subsets which may produce disease-enhancing cytokines. The present study analyses (i) the capacity of Q-Vax to induce T cell sensitization which leads to IFN-gamma responses on antigen stimulation, and (ii) the immunomodulatory, (down-regulatory) effects of the Phase I lipopolysaccharide (LPS) of the organism, which interacts with monocyte/macrophages to limit IL-2 production and production of IFN-gamma by sensitized T lymphocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., MARMION B. P. Protective or neutralizing antibody in Q fever. Am J Hyg. 1957 Sep;66(2):173–195. doi: 10.1093/oxfordjournals.aje.a119894. [DOI] [PubMed] [Google Scholar]
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Ascher M. S., Berman M. A., Ruppanner R. Initial clinical and immunologic evaluation of a new phase I Q fever vaccine and skin test in humans. J Infect Dis. 1983 Aug;148(2):214–222. doi: 10.1093/infdis/148.2.214. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Gajdosová E., Brezina R. Cell-mediated immune response to Coxiella burnetii antigens in Q fever convalescents and vaccinees. Acta Virol. 1989 Sep;33(5):474–481. [PubMed] [Google Scholar]
- Goodwin J. S. Immunologic effects of nonsteroidal anti-inflammatory agents. Med Clin North Am. 1985 Jul;69(4):793–804. doi: 10.1016/s0025-7125(16)31019-7. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Smale G., Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989 Aug;140(2):396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986 Apr;52(1):337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A. A., Marmion B. P., Hackstadt T. Analysis of the cells involved in the lymphoproliferative response to Coxiella burnetii antigens. Clin Exp Immunol. 1991 Jul;85(1):98–108. doi: 10.1111/j.1365-2249.1991.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A. A., Marmion B. P., Worswick D. A. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J Infect Dis. 1988 Apr;157(4):781–789. doi: 10.1093/infdis/157.4.781. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. M., Nicholson J. K., Holman R. C., Hubbard M. Comparison of monocyte separation methods using flow cytometric analysis. J Immunol Methods. 1989 Dec 20;125(1-2):41–47. doi: 10.1016/0022-1759(89)90076-8. [DOI] [PubMed] [Google Scholar]
- Kazãr J., Brezina R., Mayer V. Study on the effect of cyclophosphamide on experimental rickettsial infection. Acta Virol. 1971 Nov;15(6):499–509. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Rozmiarek H., Larson E. W. Experimental Q fever infection in congenitally athymic nude mice. Infect Immun. 1978 Oct;22(1):69–71. doi: 10.1128/iai.22.1.69-71.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., Williams J. C., Goodwin J. S. Cellular immunity in Q fever: specific lymphocyte unresponsiveness in Q fever endocarditis. J Infect Dis. 1985 Dec;152(6):1283–1289. doi: 10.1093/infdis/152.6.1283. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Stoker N. G., Grant K. A., Handzel Z. T., Hussain R., McAdam K. P., Dockrell H. M. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect Immun. 1989 Aug;57(8):2475–2480. doi: 10.1128/iai.57.8.2475-2480.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue K. H., Lauener R. P., Winchester R. J., Geha R. S., Vercelli D. Engagement of CD14 on human monocytes terminates T cell proliferation by delivering a negative signal to T cells. J Immunol. 1991 Aug 15;147(4):1134–1138. [PubMed] [Google Scholar]
- Lynn W. A., Golenbock D. T. Lipopolysaccharide antagonists. Immunol Today. 1992 Jul;13(7):271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- Marmion B. P., Ormsbee R. A., Kyrkou M., Wright J., Worswick D. A., Izzo A. A., Esterman A., Feery B., Shapiro R. A. Vaccine prophylaxis of abattoir-associated Q fever: eight years' experience in Australian abattoirs. Epidemiol Infect. 1990 Apr;104(2):275–287. doi: 10.1017/s0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- McDonald E. M., Mann A. H., Thomas H. C. Interferons as mediators of psychiatric morbidity. An investigation in a trial of recombinant alpha-interferon in hepatitis-B carriers. Lancet. 1987 Nov 21;2(8569):1175–1178. doi: 10.1016/s0140-6736(87)91319-5. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Phipps R. P., Stein S. H., Roper R. L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991 Oct;12(10):349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Turco J., Thompson H. A., Winkler H. H. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun. 1984 Sep;45(3):781–783. doi: 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



