Abstract
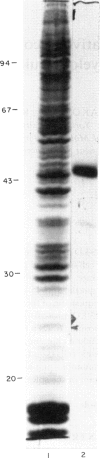
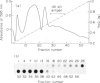
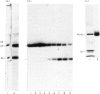
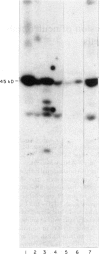
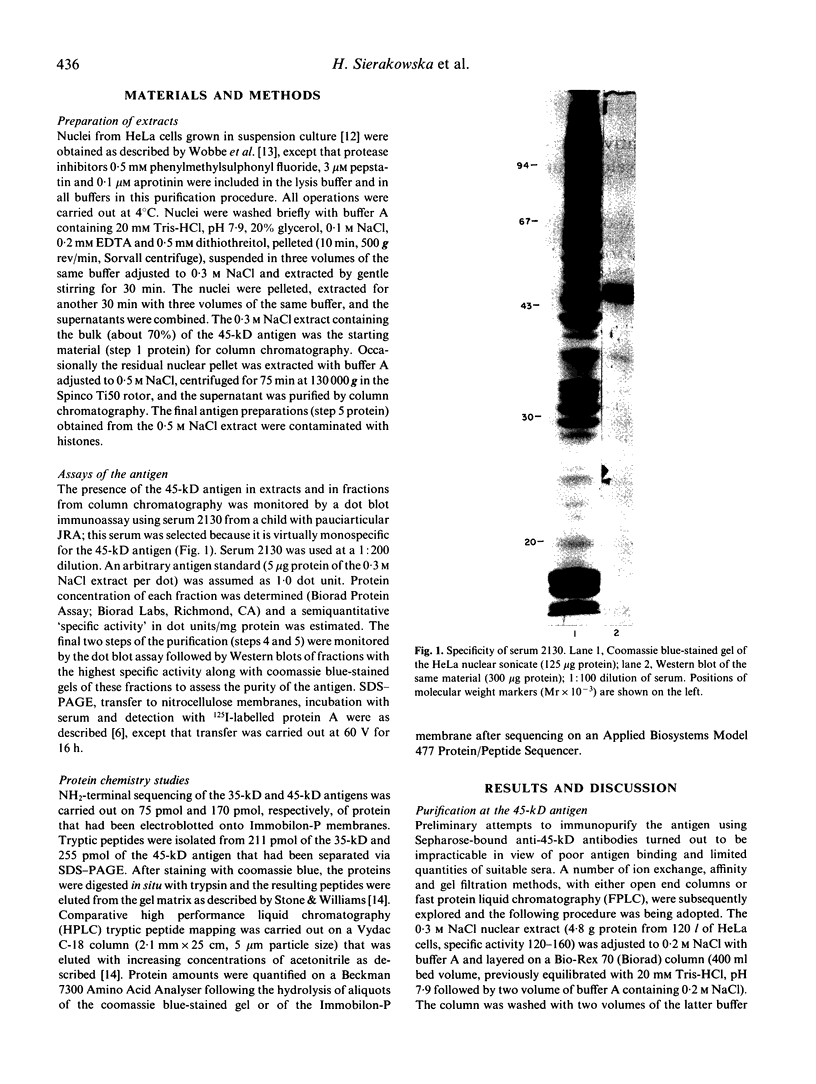
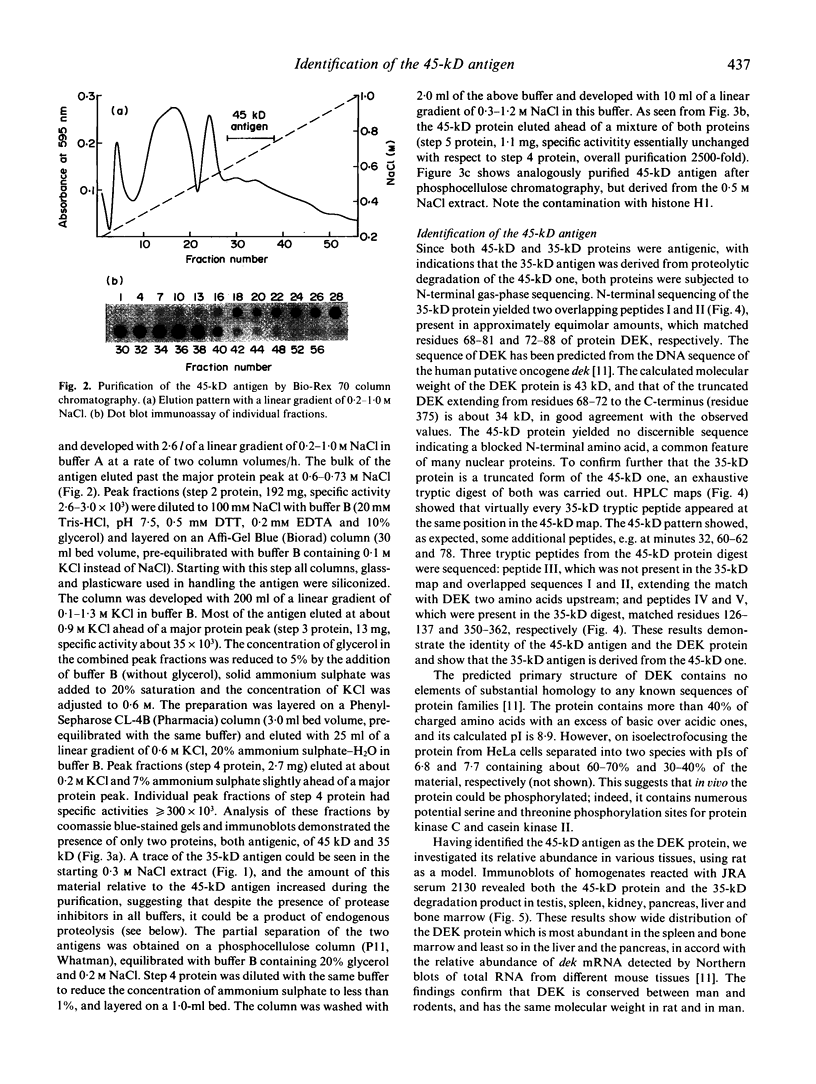
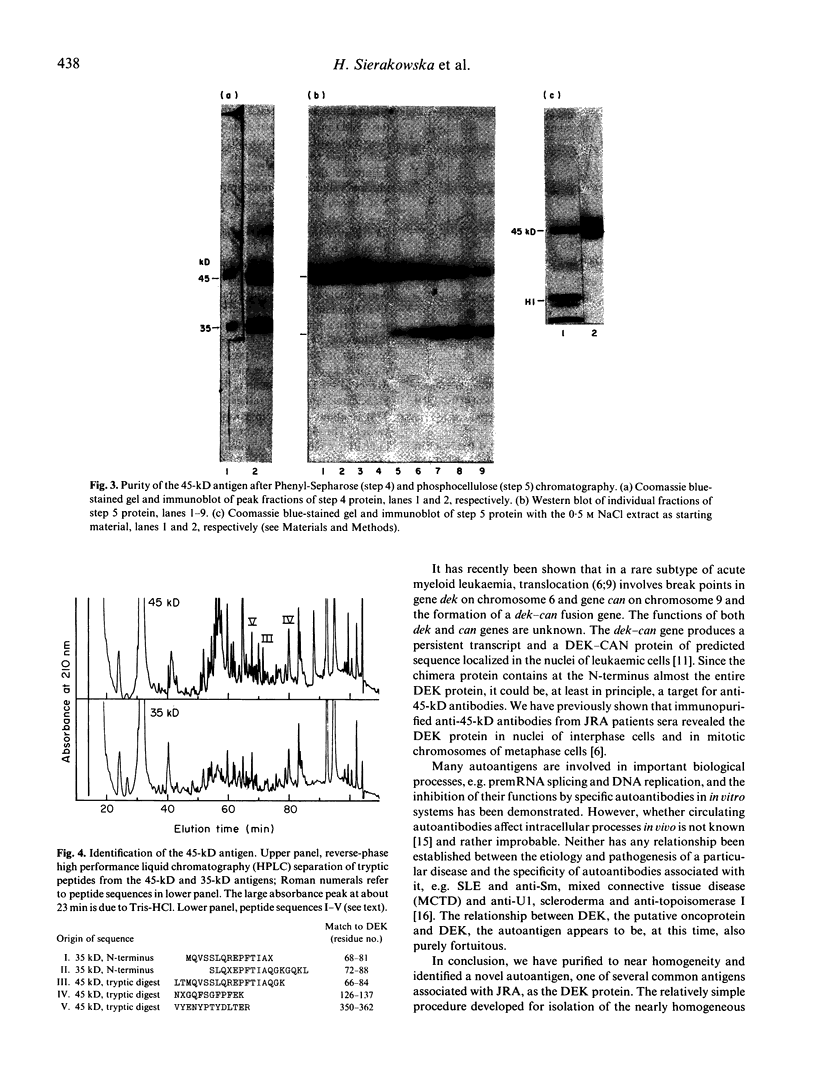
The 45-kD autoantigen associated with juvenile rheumatoid arthritis (JRA) has been isolated from HeLa cell nuclei and purified about 2500-fold to near homogeneity in a five-step chromatographic procedure. Purification of the antigen was monitored by immunoblot assays using a nearly monospecific anti-45-kD serum from a child with JRA. Tryptic peptide mapping and partial amino acid sequencing of the purified 45-kD antigen demonstrated its identity with the DEK protein. DEK is a 43-kD protein of unknown function expressed by the putative oncogene dek located on chromosome 6. As a result of a (6;9) translocation offociated with a rare subtype of acute myeloid leukaemia a chimeric protein containing most of DEK amino acids at the N-terminus is found in leukaemic cells (von Linden et al., Mol Cell Biol. 1992; 12: 1687-97). The 43-kD DEK was detected by immunoblotting with serum from a patient with JRA in a variety of rat tissues, and was most abundant in the spleen and in bone marrow.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haber P. L., Osborn T. G., Moore T. L. Antinuclear antibody in juvenile rheumatoid arthritis sera reacts with 50-40 kDa antigen(s) found in HeLa nuclear extracts. J Rheumatol. 1989 Jul;16(7):949–954. [PubMed] [Google Scholar]
- Kumar A., Williams K. R., Szer W. Purification and domain structure of core hnRNP proteins A1 and A2 and their relationship to single-stranded DNA-binding proteins. J Biol Chem. 1986 Aug 25;261(24):11266–11273. [PubMed] [Google Scholar]
- Lang B. A., Shore A. A review of current concepts on the pathogenesis of juvenile rheumatoid arthritis. J Rheumatol Suppl. 1990 Mar;21:1–15. [PubMed] [Google Scholar]
- Malleson P., Petty R. E., Fung M., Candido E. P. Reactivity of antinuclear antibodies with histones and other antigens in juvenile rheumatoid arthritis. Arthritis Rheum. 1989 Jul;32(7):919–923. [PubMed] [Google Scholar]
- Monestier M., Losman J. A., Fasy T. M., Debbas M. E., Massa M., Albani S., Bohm L., Martini A. Antihistone antibodies in antinuclear antibody-positive juvenile arthritis. Arthritis Rheum. 1990 Dec;33(12):1836–1841. doi: 10.1002/art.1780331212. [DOI] [PubMed] [Google Scholar]
- Neuer G., Bustin M., Michels H., Truckenbrodt H., Bautz F. A. Autoantibodies to the chromosomal protein HMG-17 in juvenile rheumatoid arthritis. Arthritis Rheum. 1992 Apr;35(4):472–475. doi: 10.1002/art.1780350418. [DOI] [PubMed] [Google Scholar]
- Pauls J. D., Silverman E., Laxer R. M., Fritzler M. J. Antibodies to histones H1 and H5 in sera of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1989 Jul;32(7):877–883. [PubMed] [Google Scholar]
- Southwood T. R., Malleson P. N. Antinuclear antibodies and juvenile chronic arthritis (JCA): search for a specific autoantibody associated with JCA. Ann Rheum Dis. 1991 Sep;50(9):595–598. doi: 10.1136/ard.50.9.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Sierakowska H., Szer I. S. Antinuclear antibody profile in juvenile rheumatoid arthritis. J Rheumatol. 1991 Mar;18(3):401–408. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Do autoantibodies inhibit function of their cognate antigens in vivo? Arthritis Rheum. 1989 Jul;32(7):924–925. [PubMed] [Google Scholar]
- Wittemann B., Neuer G., Michels H., Truckenbrodt H., Bautz F. A. Autoantibodies to nonhistone chromosomal proteins HMG-1 and HMG-2 in sera of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1990 Sep;33(9):1378–1383. doi: 10.1002/art.1780330910. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuklys K. L., Szer I. S., Szer W. Autoantibodies to DNA topoisomerase II in juvenile rheumatoid arthritis. Clin Exp Immunol. 1991 May;84(2):245–249. doi: 10.1111/j.1365-2249.1991.tb08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lindern M., Fornerod M., van Baal S., Jaegle M., de Wit T., Buijs A., Grosveld G. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol. 1992 Apr;12(4):1687–1697. doi: 10.1128/mcb.12.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]