Abstract
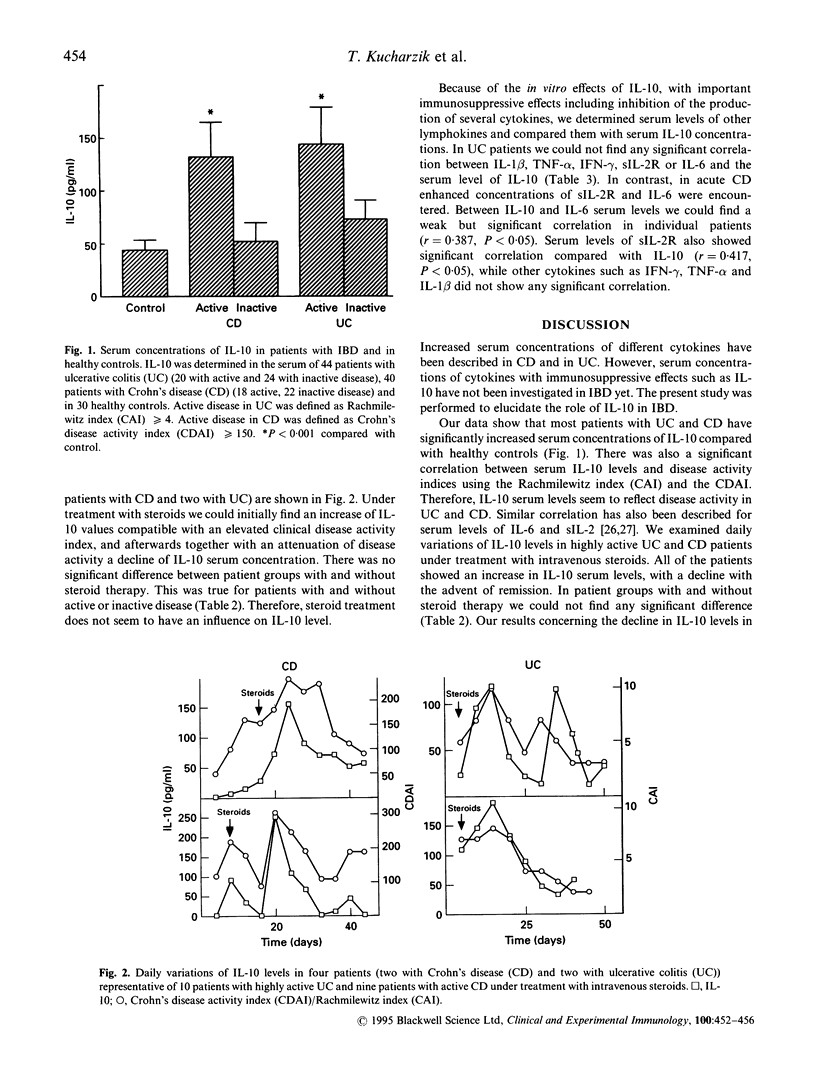
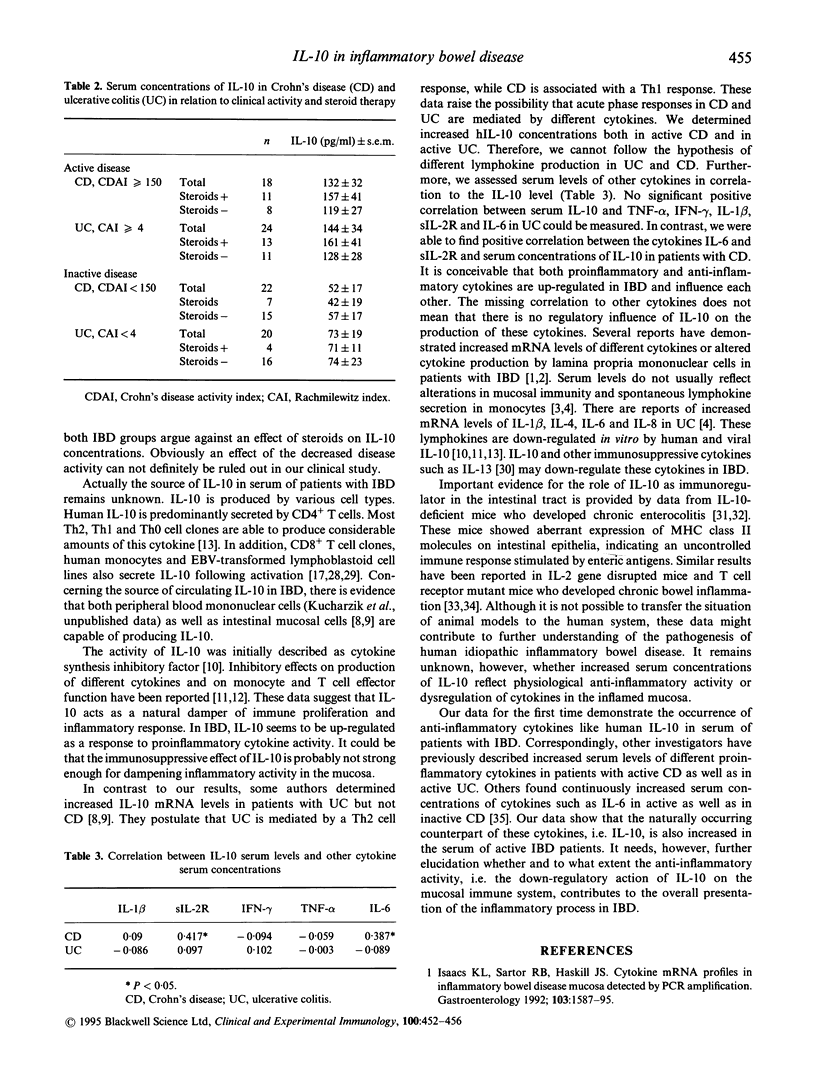
IBD is characterized by increased serum concentrations of different cytokines. IL-10 inhibits the production of proinflammatory cytokines such as IL-1, tumour necrosis factor-alpha (TNF-a), interferon-gamma (IFN-gamma) and IL-6 through inhibitory action on Th1 cells and macrophages, and it is thought to be a suppressor type cytokine. In the present study we determined serum concentrations of IL-10 in patients with ulcerative colitis (UC) and Crohn's disease (CD). We measured human IL-10 by our own newly established ELISA system using PharMingen antibodies. Serum antibodies were assessed in 44 patients with UC, 40 patients with CD, and in 30 healthy controls. Human IL-10 serum levels were significantly increased in patients with active UC (144 +/- 34 pg/ml (mean +/- s.e.m.), P < 0.001) and in active CD (132 +/- 32 pg/ml, P < 0.001) compared with healthy controls (44 +/- 9.5 pg/ml). Only patients with active CD and active UC presented with significantly increased IL-10 serum levels, while patients with inactive disease did not show any significant increase. There was no statistically significant difference between IL-10 serum levels in patients with CD or UC. Compared with clinical disease activity indices there was a significant correlation between IL-10 serum concentration and CDAI in patients with CD (r = 0.45, P < 0.01) and CAI in UC patients (r = 0.39, P < 0.05). Comparing IL-10 serum levels with serum concentrations of other proinflammatory cytokines there was a significant correlation to serum levels of sIL-2R (r = 0.417, P < 0.05) and IL-6 (r = 0.387, P < 0.05) in patients with CD. Serum cytokine levels in patients with UC did not show any significant correlation to IL-10 serum concentration. IL-10 is elevated in serum of patients with active CD and UC, suggesting that IL-10 acts as a naturally occurring damper in the acute inflammatory process of IBD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blay J. Y., Burdin N., Rousset F., Lenoir G., Biron P., Philip T., Banchereau J., Favrot M. C. Serum interleukin-10 in non-Hodgkin's lymphoma: a prognostic factor. Blood. 1993 Oct 1;82(7):2169–2174. [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Juby L. D., Heatley R. V., Lobo A. J., Bullimore D. W., Axon A. T. Soluble interleukin-2 receptor in Crohn's disease: relation of serum concentrations to disease activity. Gut. 1990 Sep;31(9):1033–1036. doi: 10.1136/gut.31.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Gotlieb W. H., Abrams J. S., Watson J. M., Velu T. J., Berek J. S., Martínez-Maza O. Presence of interleukin 10 (IL-10) in the ascites of patients with ovarian and other intra-abdominal cancers. Cytokine. 1992 Sep;4(5):385–390. doi: 10.1016/1043-4666(92)90082-3. [DOI] [PubMed] [Google Scholar]
- Gross V., Andus T., Caesar I., Roth M., Schölmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn's disease. Gastroenterology. 1992 Feb;102(2):514–519. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., Moore K. W., Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992 May;4(5):563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Hyams J. S., Fitzgerald J. E., Treem W. R., Wyzga N., Kreutzer D. L. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993 May;104(5):1285–1292. doi: 10.1016/0016-5085(93)90336-b. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Gallagher A., Kurlak L., Hawkey C. J. Plasma and tissue interleukin-2 receptor levels in inflammatory bowel disease. Clin Exp Immunol. 1990 Oct;82(1):75–80. doi: 10.1111/j.1365-2249.1990.tb05406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A., Devière J., Byl B., De Groote D., Vincent J. L., Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994 Mar 19;343(8899):707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mueller C., Knoflach P., Zielinski C. C. T-cell activation in Crohn's disease. Increased levels of soluble interleukin-2 receptor in serum and in supernatants of stimulated peripheral blood mononuclear cells. Gastroenterology. 1990 Mar;98(3):639–646. [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Peyron F., Burdin N., Ringwald P., Vuillez J. P., Rousset F., Banchereau J. High levels of circulating IL-10 in human malaria. Clin Exp Immunol. 1994 Feb;95(2):300–303. doi: 10.1111/j.1365-2249.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989 Jan 14;298(6666):82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Strober W., Ehrhardt R. O. Chronic intestinal inflammation: an unexpected outcome in cytokine or T cell receptor mutant mice. Cell. 1993 Oct 22;75(2):203–205. doi: 10.1016/0092-8674(93)80062-j. [DOI] [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman K. R., Simon P. L., West G. A., Cominelli F., Rachmilewitz D., Klein J. S., Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993 Mar;104(3):749–758. doi: 10.1016/0016-5085(93)91010-f. [DOI] [PubMed] [Google Scholar]
- Zurawski G., de Vries J. E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994 Jan;15(1):19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]



