Abstract
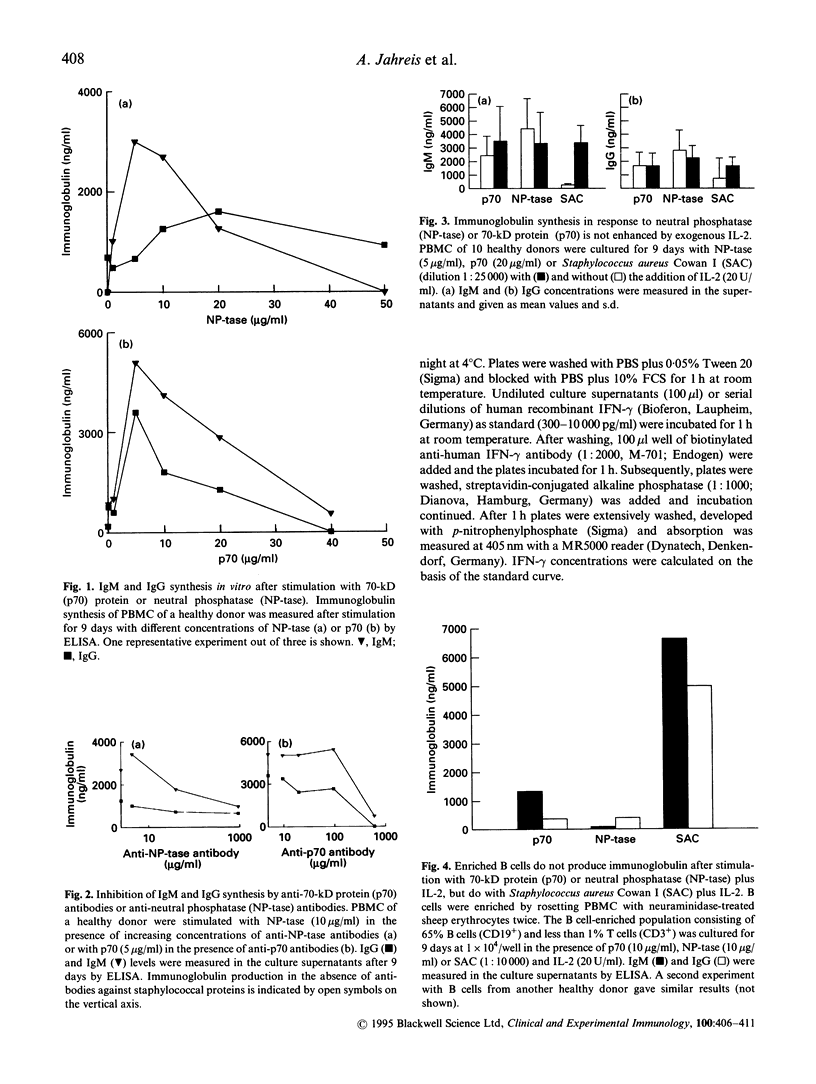
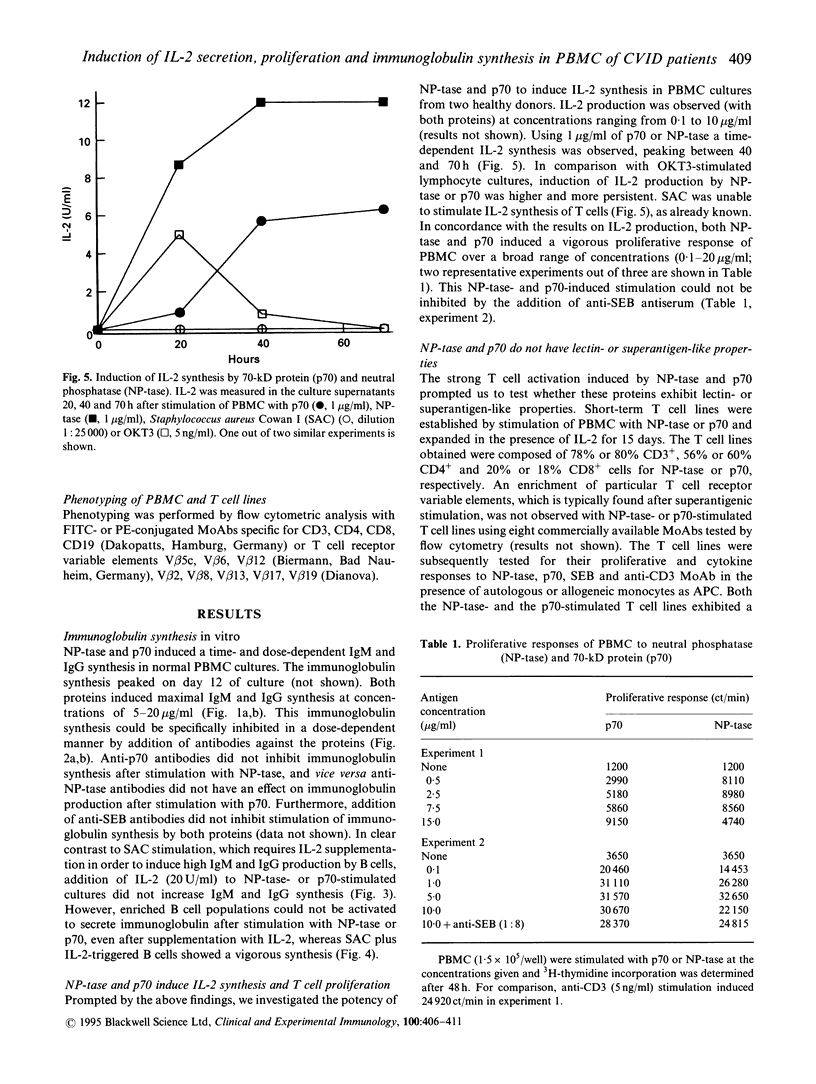
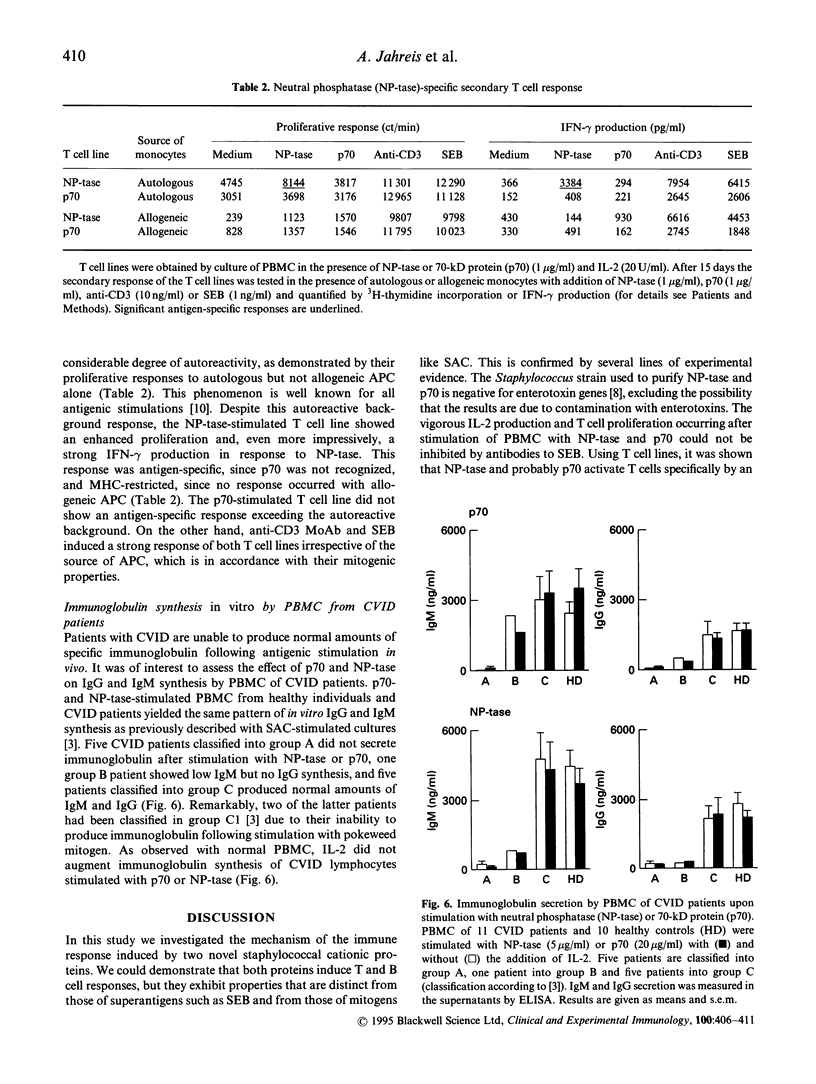
Two cationic proteins, a neutral phosphatase (NP-tase) and a 70-kD protein (p70) were isolated from Staphylococcus aureus by ion exchange chromatography. We compared their properties to those of the well established B cell mitogen of whole, fixed Staph. aureus strain Cowan I cells (SAC). Both purified proteins were able to induce immunoglobulin synthesis in PBMC cultures of healthy donors. NP-tase and p70 also induced immunoglobulin synthesis of PBMC from those patients with CVID who were also responsive to SAC plus IL-2 stimulation. Immunoglobulin synthesis in response to NP-tase and to p70 was time- and dose-dependent and could be inhibited by addition of specific antibodies against the proteins. In contrast to SAC, no addition of exogenous IL-2 was necessary to obtain maximal immunoglobulin synthesis induced by NP-tase or p70. However, neither protein was able to induce immunoglobulin synthesis in B cell-enriched cultures. High amounts of IL-2 were found in supernatants of PBMC from healthy donors following stimulation with low concentrations of NP-tase or p70, and this was associated with vigorous lymphocyte proliferation. Both proteins behave like typical antigens, and not like lectins or superantigens, since an NP-tase-stimulated T cell line showed an antigen-specific, MHC-restricted secondary response. In addition, no preferential T cell receptor V beta chain usage was found with eight V beta-specific MoAb. It is likely that the two proteins induce antigen-specific T cell activation, which is then followed by polyclonal activation of B cells via CD40 receptors and cytokine release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant A., Calver N. C., Toubi E., Webster A. D., Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990 Aug;56(2):239–248. doi: 10.1016/0090-1229(90)90145-g. [DOI] [PubMed] [Google Scholar]
- Finnegan A., Needleman B. W., Hodes R. J. Function of autoreactive T cells in immune responses. Immunol Rev. 1990 Aug;116:15–31. doi: 10.1111/j.1600-065x.1990.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Hartwig U. T-lymphocyte stimulation by microbial superantigens. Chem Immunol. 1992;55:36–64. [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Lipsky P. E. The role of CD40-CD40 ligand interaction in human T cell-B cell collaboration. J Immunol. 1994 Aug 1;153(3):1027–1036. [PubMed] [Google Scholar]
- Rump J. A., Jahreis A., Schlesier M., Dräger R., Melchers I., Peter H. H. Possible role of IL-2 deficiency for hypogammaglobulinaemia in patients with common variable immunodeficiency. Clin Exp Immunol. 1992 Aug;89(2):204–210. doi: 10.1111/j.1365-2249.1992.tb06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Shokri F., Mageed R. A., Maziak B. R., Jefferis R. Expression of VHIII-associated cross-reactive idiotype on human B lymphocytes. Association with staphylococcal protein A binding and Staphylococcus aureus Cowan I stimulation. J Immunol. 1991 Feb 1;146(3):936–940. [PubMed] [Google Scholar]
- Stohl W., Elliott J. E., Linsley P. S. Human T cell-dependent B cell differentiation induced by staphylococcal superantigens. J Immunol. 1994 Jul 1;153(1):117–127. [PubMed] [Google Scholar]
- Timmerman C. P., Mattsson E., Martinez-Martinez L., De Graaf L., Van Strijp J. A., Verbrugh H. A., Verhoef J., Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993 Oct;61(10):4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif Y., Schiltz E., Okada K., Batsford S., Vogt A. Staphylococcal neutral phosphatase. A highly cationic molecule with binding properties for immunoglobulin. APMIS. 1994 Dec;102(12):891–900. [PubMed] [Google Scholar]



