Abstract
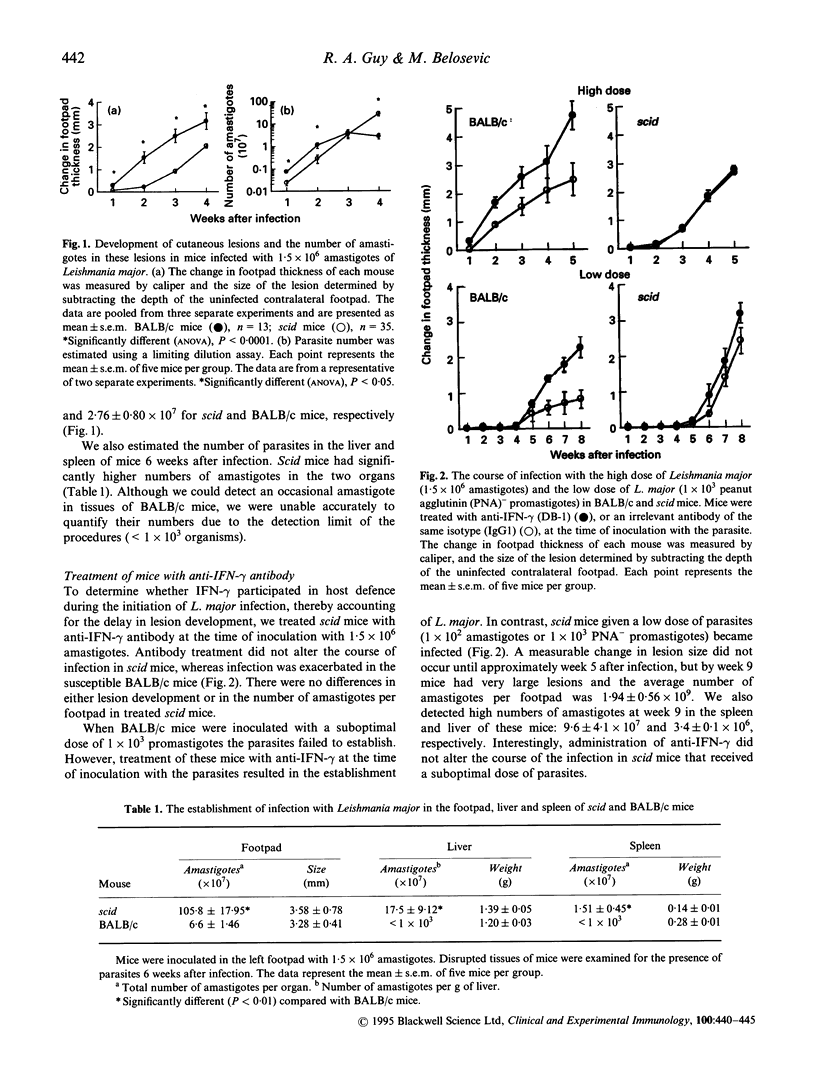
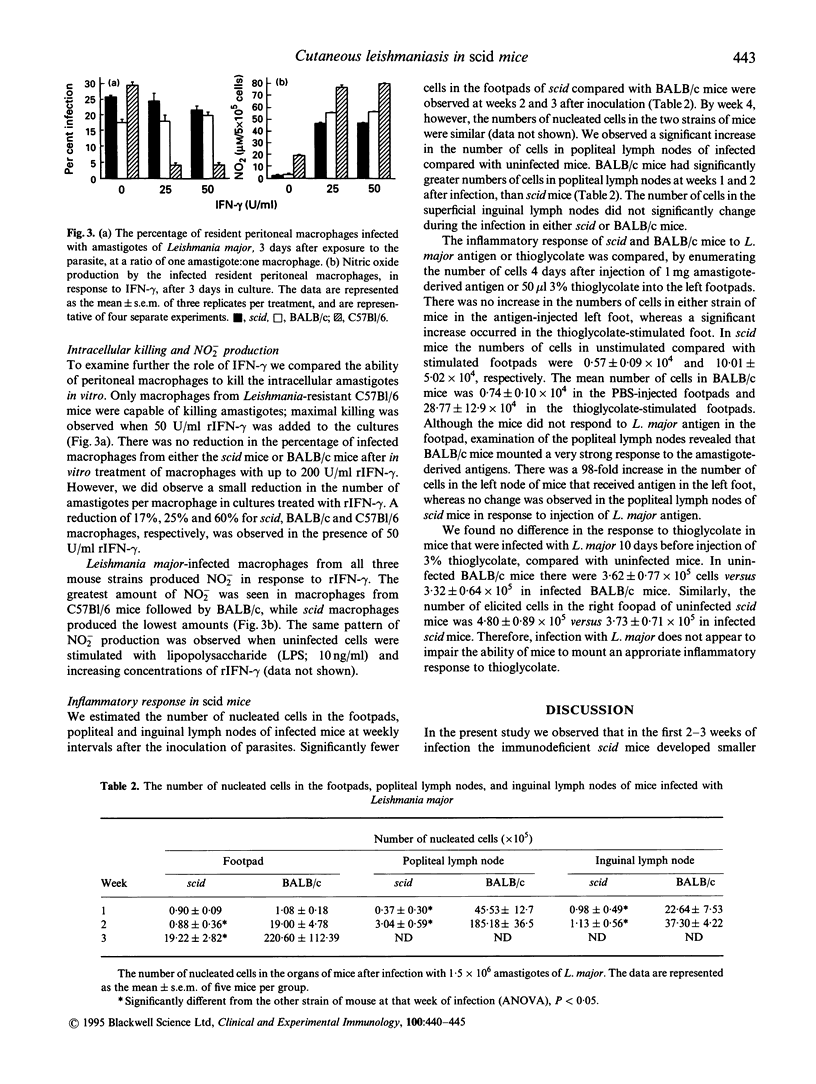
The initiation of Leishmania major infection in susceptible BALB/c mice is regulated by interferon-gamma (IFN-gamma). To examine further the mechanisms of IFN-gamma-dependent regulation of the establishment of L. major, we studied the characteristics of the infection in severe combined immunodeficient (scid) mice. In the first 2 weeks of infection, we observed a delay in the development of the lesions in the footpads and lower numbers of parasites in scid compared with BALB/c mice. By week 5 after infection, the size of the leishmanial lesion was similar in both strains of mice, but the number of parasites in scid mice was 100-fold higher than in BALB/c. Treatment with anti-IFN-gamma during the establishment of L. major did not alter the course of infection in scid mice, while it exacerbated lesion development in BALB/c mice. Macrophages from scid mice were unable to kill L. major when stimulated with IFN-gamma in vitro, and produced lower levels of nitric oxide compared with macrophages from susceptible BALB/c or the resistant C57Bl/6 mice. We examined whether delayed lesion development in scid mice was due to their inability to mount appropriate inflammatory responses. While significantly fewer nucleated cells were present in the footpads of scid mice compared with BALB/c, 2 and 3 weeks after infection, no difference in inflammatory response between scid and BALB/c mice was observed in response to L. major antigen in the footpads. In contrast, there was a dramatic increase in the number of cells in the popliteal lymph nodes of BALB/c mice. Decreased inflammatory responses of scid mice in the footpad (at the site of infection) may contribute to slower development of leishmanial lesions during the first 2 weeks of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Meltzer M. S., Nacy C. A. IL-2. A cofactor for induction of activated macrophage resistance to infection. J Immunol. 1990 Aug 1;145(3):831–839. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A., Wei G., Menon J. N., Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992 Jul 24;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Gordon J. R., Burd P. R., Galli S. J. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990 Dec;11(12):458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- Green S. J., Nacy C. A., Meltzer M. S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991 Jul;50(1):93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Jackson P. R., Pappas M. G., Hansen B. D. Fluorogenic substrate detection of viable intracellular and extracellular pathogenic protozoa. Science. 1985 Jan 25;227(4685):435–438. doi: 10.1126/science.2578226. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Bancroft G. J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992 Oct;60(10):4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W. C., Bennett M., Pakes S. P., Kumar V., Steutermann D., Owusu I., Mikhael A. Resistance to Mycoplasma pulmonis mediated by activated natural killer cells. J Infect Dis. 1990 Jun;161(6):1269–1275. doi: 10.1093/infdis/161.6.1269. [DOI] [PubMed] [Google Scholar]
- Leiby D. A., Schreiber R. D., Nacy C. A. IFN-gamma produced in vivo during the first two days is critical for resolution of murine Leishmania major infections. Microb Pathog. 1993 Jun;14(6):495–500. doi: 10.1006/mpat.1993.1049. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Moss D., Parkinson C., Rogers M. V., Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991 Dec;21(12):3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. J Immunogenet. 1983 Oct;10(5):395–412. doi: 10.1111/j.1744-313x.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr Opin Immunol. 1991 Feb;3(1):49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- Ridel P. R., Esterre P., Dedet J. P., Pradinaud R., Santoro F., Capron A. Killer cells in human cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1988;82(2):223–226. doi: 10.1016/0035-9203(88)90419-1. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–3155. [PubMed] [Google Scholar]
- Sher A., Oswald I. P., Hieny S., Gazzinelli R. T. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993 May 1;150(9):3982–3989. [PubMed] [Google Scholar]
- Stenger S., Thüring H., Röllinghoff M., Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994 Sep 1;180(3):783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Varkila K., Chatelain R., Leal L. M., Coffman R. L. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type-1 response and renders them capable of healing Leishmania major infection. Eur J Immunol. 1993 Jan;23(1):262–268. doi: 10.1002/eji.1830230141. [DOI] [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva R., Sacks D. L. Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infect Immun. 1987 Nov;55(11):2802–2806. doi: 10.1128/iai.55.11.2802-2806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



