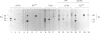Abstract
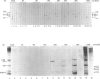
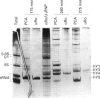
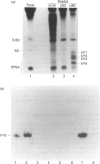
Anti-Ro sera immunoprecipitate Ro ribonucleoproteins (RNPs) from human cell extracts. Ro RNPs are biochemically heterogeneous particles whose functions are unknown and whose exact composition remains controversial. In addition to 60-kD Ro and to La proteins, a 52-kD polypeptide (p52) has been proposed to be a stable component of the Ro RNPs. To confirm the immunological studies supporting this hypothesis, we have biochemically purified Ro RNPs from HeLa cells using non-denaturing conditions. Ro RNPs segregated into three distinct populations, one of which only contained hY5 RNA (RohY5 RNPs). No p52 co-purified with Ro RNPs. Despite the absence of p52, purified Ro RNPs had biochemical and immunological properties identical to those of unfractionated Ro RNPs. Many anti-Ro sera only recognize p52 in immunoblots, and are said to be monospecific anti-p52. Preincubation with purified RohY5 RNPs (free of p52) of all human anti-Ro (including so-called monospecific anti-p52) sera abolished their capacity to immunoprecipitate Ro RNPs from unfractionated HeLa cell extracts. Conversely, preincubation of anti-Ro sera with purified p52 protein specifically inhibited recognition of p52 in immunoblots, but did not interfere with immunoprecipitation of Ro RNPs. Our data demonstrate that anti-p52 antibodies do not target intact Ro RNPs, nor do they target the native 60-kD Ro protein. Contrary to previous reports, p52 protein is not a stable component of antigenically intact Ro RNPs.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Arnett F. C., Provost T. T., Stevens M. B. Sjögren's syndrome: association of anti-Ro(SS-A) antibodies with vasculitis, hematologic abnormalities, and serologic hyperreactivity. Ann Intern Med. 1983 Feb;98(2):155–159. doi: 10.7326/0003-4819-98-2-155. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Craft J. Biochemical and immunological heterogeneity of the Ro ribonucleoprotein particles. Analysis with sera specific for the RohY5 particle. J Clin Invest. 1989 Jul;84(1):270–279. doi: 10.1172/JCI114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Craft J. Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Invest. 1990 Apr;85(4):1182–1190. doi: 10.1172/JCI114551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Lopez-Longo F. J., Lapointe S., Ménard H. A. Sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum. 1991 Jun;34(6):722–730. doi: 10.1002/art.1780340613. [DOI] [PubMed] [Google Scholar]
- Boire G., Ménard H. A., Gendron M., Lussier A., Myhal D. Rheumatoid arthritis: anti-Ro antibodies define a non-HLA-DR4 associated clinicoserological cluster. J Rheumatol. 1993 Oct;20(10):1654–1660. [PubMed] [Google Scholar]
- Buyon J. P., Slade S. G., Chan E. K., Tan E. M., Winchester R. Effective separation of the 52 kDa SSA/Ro polypeptide from the 48 kDa SSB/La polypeptide by altering conditions of polyacrylamide gel electrophoresis. J Immunol Methods. 1990 May 25;129(2):207–210. doi: 10.1016/0022-1759(90)90440-7. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K., Andrade L. E. Antinuclear antibodies in Sjögren's syndrome. Rheum Dis Clin North Am. 1992 Aug;18(3):551–570. [PubMed] [Google Scholar]
- Chan E. K., Hamel J. C., Buyon J. P., Tan E. M. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest. 1991 Jan;87(1):68–76. doi: 10.1172/JCI115003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 1989 Mar;8(3):841–850. doi: 10.1002/j.1460-2075.1989.tb03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Itoh Y., Frank M. B. Protein heterogeneity in the human Ro/SSA ribonucleoproteins. The 52- and 60-kD Ro/SSA autoantigens are encoded by separate genes. J Clin Invest. 1991 Jan;87(1):177–186. doi: 10.1172/JCI114968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Itoh K., Frank M. B., Reichlin M. Autoantibodies to the Ro/SSA autoantigen are conformation dependent. II: Antibodies to the denatured form of 52 kD Ro/SSA are a cross reacting subset of antibodies to the native 60 kD Ro/SSA molecule. Autoimmunity. 1992;14(2):89–95. doi: 10.3109/08916939209083125. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Reichlin M. Autoantibodies to the Ro/SSA antigen are conformation dependent. I: Anti-60 kD antibodies are mainly directed to the native protein; anti-52 kD antibodies are mainly directed to the denatured protein. Autoimmunity. 1992;14(1):57–65. doi: 10.3109/08916939309077357. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Reichlin M. Ro/SS-A antigen in human platelets. Different distributions of the isoforms of Ro/SS-A protein and the Ro/SS-A-binding RNA. Arthritis Rheum. 1991 Jul;34(7):888–893. doi: 10.1002/art.1780340715. [DOI] [PubMed] [Google Scholar]
- James J. A., Dickey W. D., Fujisaku A., O'Brien C. A., Deutscher S. L., Keene J. D., Harley J. B. Antigenicity of a recombinant Ro (SS-A) fusion protein. Arthritis Rheum. 1990 Jan;33(1):102–106. doi: 10.1002/art.1780330114. [DOI] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Kelekar A., Saitta M. R., Keene J. D. Molecular composition of Ro small ribonucleoprotein complexes in human cells. Intracellular localization of the 60- and 52-kD proteins. J Clin Invest. 1994 Apr;93(4):1637–1644. doi: 10.1172/JCI117145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Newkirk M. M., Capra J. D., Sontheimer R. D. Molecular characterization of human Ro/SS-A antigen. Amino terminal sequence of the protein moiety of human Ro/SS-A antigen and immunological activity of a corresponding synthetic peptide. J Clin Invest. 1988 Jul;82(1):96–101. doi: 10.1172/JCI113607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamula M. J., O'Brien C. A., Harley J. B., Hardin J. A. The Ro ribonucleoprotein particle: induction of autoantibodies and the detection of Ro RNAs among species. Clin Immunol Immunopathol. 1989 Sep;52(3):435–446. doi: 10.1016/0090-1229(89)90158-x. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Silverman E. D., Laxer R. M., Bentur L., Isacovics B., Hardin J. A. Human monoclonal anti-La antibodies. The La protein resides on a subset of Ro particles. J Immunol. 1989 Nov 1;143(9):2923–2928. [PubMed] [Google Scholar]
- McCauliffe D. P., Lux F. A., Lieu T. S., Sanz I., Hanke J., Newkirk M. M., Bachinski L. L., Itoh Y., Siciliano M. J., Reichlin M. Molecular cloning, expression, and chromosome 19 localization of a human Ro/SS-A autoantigen. J Clin Invest. 1990 May;85(5):1379–1391. doi: 10.1172/JCI114582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauliffe D. P., Sontheimer R. D. Molecular characterization of the Ro/SS-A autoantigens. J Invest Dermatol. 1993 Jan;100(1):73S–79S. doi: 10.1111/1523-1747.ep12355637. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilof J. F., Bantjes I., De Jong J., Van Dam A. P., Smeenk R. J. The detection of anti-Ro/SS-A and anti-La/SS-B antibodies. A comparison of counterimmunoelectrophoresis with immunoblot, ELISA, and RNA-precipitation assays. J Immunol Methods. 1990 Oct 19;133(2):215–226. doi: 10.1016/0022-1759(90)90362-y. [DOI] [PubMed] [Google Scholar]
- Mond C. B., Peterson M. G., Rothfield N. F. Correlation of anti-Ro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis Rheum. 1989 Feb;32(2):202–204. doi: 10.1002/anr.1780320213. [DOI] [PubMed] [Google Scholar]
- O'Brien C. A., Harley J. B. A subset of hY RNAs is associated with erythrocyte Ro ribonucleoproteins. EMBO J. 1990 Nov;9(11):3683–3689. doi: 10.1002/j.1460-2075.1990.tb07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C. A., Wolin S. L. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994 Dec 1;8(23):2891–2903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., van Venrooij W. J. Analysis of protein--RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991 Oct 11;19(19):5173–5180. doi: 10.1093/nar/19.19.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader M. D., O'Brien C., Liu Y. S., Harley J. B., Reichlin M. Heterogeneity of the Ro/SSA antigen. Different molecular forms in lymphocytes and red blood cells. J Clin Invest. 1989 Apr;83(4):1293–1298. doi: 10.1172/JCI114014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M. Significance of the Ro antigen system. J Clin Immunol. 1986 Sep;6(5):339–348. doi: 10.1007/BF00915372. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Haselby J. A., Meilof J. F., Smeenk R. J., Unnasch T. R., Greene B. M., Hoch S. O. Characterization of the autoantigen calreticulin. J Immunol. 1991 Nov 1;147(9):3031–3039. [PubMed] [Google Scholar]
- Saitta M. R., Arnett F. C., Keene J. D. 60-kDa Ro protein autoepitopes identified using recombinant polypeptides. J Immunol. 1994 Apr 15;152(8):4192–4202. [PubMed] [Google Scholar]
- Slobbe R. L., Pluk W., van Venrooij W. J., Pruijn G. J. Ro ribonucleoprotein assembly in vitro. Identification of RNA-protein and protein-protein interactions. J Mol Biol. 1992 Sep 20;227(2):361–366. doi: 10.1016/0022-2836(92)90890-v. [DOI] [PubMed] [Google Scholar]
- Slobbe R. L., Pruijn G. J., Damen W. G., van der Kemp J. W., van Venrooij W. J. Detection and occurrence of the 60- and 52-kD Ro (SS-A) antigens and of autoantibodies against these proteins. Clin Exp Immunol. 1991 Oct;86(1):99–105. doi: 10.1111/j.1365-2249.1991.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer R. D., Maddison P. J., Reichlin M., Jordon R. E., Stastny P., Gilliam J. N. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tsuzaka K., Fujii T., Akizuki M., Mimori T., Tojo T., Fujii H., Tsukatani Y., Kubo A., Homma M. Clinical significance of antibodies to native or denatured 60-kd or 52-kd Ro/SS-A proteins in Sjögren's syndrome. Arthritis Rheum. 1994 Jan;37(1):88–92. doi: 10.1002/art.1780370113. [DOI] [PubMed] [Google Scholar]
- Whittingham S. B-cell epitopes of La and Ro autoantigens. Mol Biol Rep. 1992 Jun;16(3):175–181. doi: 10.1007/BF00464705. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]